Spatiotemporal Neural Correlates of Brain-Computer Interface
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10041.Full.Pdf
The Journal of Neuroscience, July 23, 2014 • 34(30):10041–10054 • 10041 Systems/Circuits Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders Sarah R. Heilbronner and Suzanne N. Haber Department of Pharmacology and Physiology, University of Rochester Medical Center, Rochester, New York 14642 The cingulum bundle (CB) is one of the brain’s major white matter pathways, linking regions associated with executive function, decision-making, and emotion. Neuroimaging has revealed that abnormalities in particular locations within the CB are associated with specific psychiatric disorders, including depression and bipolar disorder. However, the fibers using each portion of the CB remain unknown. In this study, we used anatomical tract-tracing in nonhuman primates (Macaca nemestrina, Macaca fascicularis, Macaca mulatta)toexaminetheorganizationofspecificcingulate,noncingulatefrontal,andsubcorticalpathwaysthroughtheCB.Thegoalswere as follows: (1) to determine connections that use the CB, (2) to establish through which parts of the CB these fibers travel, and (3) to relate the CB fiber pathways to the portions of the CB identified in humans as neurosurgical targets for amelioration of psychiatric disorders. Results indicate that cingulate, noncingulate frontal, and subcortical fibers all travel through the CB to reach both cingulate and noncin- gulate targets. However, many brain regions send projections through only part, not all, of the CB. For example, amygdala fibers are not present in the caudal portion of the dorsal CB. These results allow segmentation of the CB into four unique zones. We identify the specific connections that are abnormal in psychiatric disorders and affected by neurosurgical interventions, such as deep brain stimulation and cingulotomy. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -
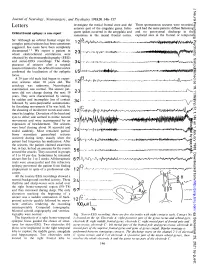
Letters Anterior Part of the Cingulate Gyrus
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.1.146 on 1 January 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:146-157 investigate the mesial frontal zone and the Three spontaneous seizures were recorded, Letters anterior part of the cingulate gyrus. Infre- each had the same pattern: diffuse flattening quent spikes occurred in the amygdala and and no in the Orbital frontal epilepsy: a case report paroxysmal discharge sometimes in the mesial frontal cortex. explored sites in the frontal or temporal Sir: Although an orbital frontal origin for A complex partial seizures has been sometimes 12 suggested, few cases have been completely documented.1 2 We report a patient in 23 whom electroclinical correlations were obtained by electroencephalography (EEG) and stereo-EEG recordings. The disap- 3.4 I-w VI,-#a+r - pearance of seizures after a surgical resection limited to the orbital frontal cortex confirmed the localisation of the epileptic 4.5 o I\A-.ViJV "fJ4 WOO focus. A 29 year old male had begun to experi- B ence seizures when 10 years old. The aetiology was unknown. Neurological examination was normal. The seizure pat- terns did not change during the next 19 23 ., years. They were characterised by staring, r by sudden and incomplete loss of contact -- - -. , A- followed by semi-purposeful automatisms, 3-4 v by thrashing movements if he was held, by the shouting of incoherent words and some- times by laughter. Deviation of the head and eyes to either side seemed to mimic natural Protected by copyright. -

Supporting Information for “Endocast Morphology of Homo Naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, S
Supporting Information for “Endocast Morphology of Homo naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, Shawn D. Hurst, Heather M. Garvin, P. Thomas Schoenemann, William B. Vanti, Lee R. Berger, and John Hawks What follows are our descriptions, illustrations, some basic interpretation, and a more specific discussion of the functional, comparative, and taxonomic issues surrounding these hominins. We use the neuroanatomical nomenclature from Duvernoy (19). DH1 Occipital The DH1 occipital fragment (Figs 1, S2) measures ca 105 mm in width between left temporo- occipital incisure and right sigmoid sinus. It is 61 mm in height on the left side, and 47 mm on the right side. The fragment covers the entire left and mostly complete right occipital lobes. The lobes are strongly asymmetrical, with the left clearly larger than the right, and more posteriorly protruding. There are faint traces of a lateral remnant of the lunate sulcus on the left side, and a dorsal bounding lunate as well (#4 and #6 in Fig 1). The right side shows a very small groove at the end of the lateral sinus, which could be a remnant of the lunate sulcus. The major flow from the longitudinal sinus is to the right. Small portions of both cerebellar lobes, roughly 15 mm in height are present. There is a suggestion of a great cerebellar sulcus on the right side. The width from the left lateral lunate impression to the midline is 43mm. The distance from left occipital pole (the most posteriorly projecting point, based on our best estimate of the proper orientation) to the mid-sagittal plane is 30 mm. -

On the Scent of Human Olfactory Orbitofrontal Cortex: Meta-Analysis and Comparison to Non-Human Primates
Brain Research Reviews 50 (2005) 287 – 304 www.elsevier.com/locate/brainresrev Review On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates Jay A. Gottfrieda,*, David H. Zaldb aDepartment of Neurology and the Cognitive Neurology and Alzheimer’s Disease Center, Northwestern University Feinberg School of Medicine, 320 E. Superior St., Searle 11-453, Chicago, IL 60611, USA bDepartment of Psychology, Vanderbilt University, Nashville, TN 37240, USA Accepted 25 August 2005 Available online 6 October 2005 Abstract It is widely accepted that the orbitofrontal cortex (OFC) represents the main neocortical target of primary olfactory cortex. In non-human primates, the olfactory neocortex is situated along the basal surface of the caudal frontal lobes, encompassing agranular and dysgranular OFC medially and agranular insula laterally, where this latter structure wraps onto the posterior orbital surface. Direct afferent inputs arrive from most primary olfactory areas, including piriform cortex, amygdala, and entorhinal cortex, in the absence of an obligatory thalamic relay. While such findings are almost exclusively derived from animal data, recent cytoarchitectonic studies indicate a close anatomical correspondence between non-human primate and human OFC. Given this cross-species conservation of structure, it has generally been presumed that the olfactory projection area in human OFC occupies the same posterior portions of OFC as seen in non-human primates. This review questions this assumption by providing a critical survey of the localization of primate and human olfactory neocortex. Based on a meta-analysis of human functional neuroimaging studies, the region of human OFC showing the greatest olfactory responsivity appears substantially rostral and in a different cytoarchitectural area than the orbital olfactory regions as defined in the monkey. -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Jaw and Orofacial Motor Representation in Cat Orbital Gyrus 593
Jpn. J. Oral Biol., 33: 592-599, 1991. ORIGINAL Jaw and orofacial motor representation in cat orbital gyrus Yoshiyuki Tsuboi, Koichi Iwata, Hiroyuki Muramatsu, Junichi Yagi, Yuji Inomata, Reo Kikuta, Keiji Yoshii and Rhyuji Sumino Department of Physiology, Nihon University. School of Dentistry, 1-8-13 Kandasurugadai, Chiyoda-ku, Tokyo 101, Japan (Director: Prof. Rhyuji Sumino) Accepted for publication: June•k 20, 1991•l Key words: motor effect/trigeminal nerve/orbital gyrus/ICMS/cat Abstract: Jaw and orofacial motor representation in the orbital cortex was studied by intracortical microstimulation (ICMS) in lightly anesthetized cats. ICMS of the posterior portion of the the orbital gyrus produced movements of facial muscles, whereas stimulation of the anterior portion of the orbital gyrus produced more generalized movement of facial, jaw and tongue muscles. The region producing jaw movements was more restricted than the regions producing tongue and facial movements. Repe- titive stimulation of the anterior orbital gyrus produced either rhythmic jaw movements or sustained jaw opening. Cytoarchitectonically, the posterior portion of the orbital gyrus was restricted to area 43 and the anterior portion to areas 43 and 6 aƒÀ. present study, the function of the orbital gyrus Introduction was studied by detailed topographic mapping The orbital cortex in the cat has been ter- of the effects produced by ICMS and the med the cortical masticatory area because results correlated with the cytoarchitectonic criteria of Hassler and Muhs-Clement7). repetitive stimulation of its anterior part pro- duces rhythmic jaw and tongue move- Materials and Methods ments1-3). The axons of cells in this cortical region may project to a "pattern generator" Experiments were performed on 16 cats in the rhythmic generation of masticatory anesthetized initially with Ketamine HCL (50 jaw movements2,4). -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -

Normal Cortical Anatomy
Normal Cortical Anatomy MGH Massachusetts General Hospital Harvard Medical School NORMAL CORTICAL ANATOMY • Sagittal • Axial • Coronal • The Central Sulcus NP/MGH Sagittal Neuroanatomy NP/MGH Cingulate sulcus Superior frontal gyrus Marginal ramus of Cingulate sulcus Cingulate gyrus Paracentral lobule Superior parietal lobule Parietooccipital sulcus Cuneus Calcarine sulcus Lingual gyrus Subcallosal gyrus Gyrus rectus Fastigium, fourth ventricle NP/MGH Superior frontal gyrus Cingulate sulcus Precentral gyrus Marginal ramus of Cingulate gyrus Central sulcus Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Cuneus Calcarine sulcus Frontomarginal gyrus Lingual gyrus Caudothallamic groove Gyrus rectus NP/MGH Precentral sulcus Central sulcus Superior frontal gyrus Marginal ramus of Corona radiata Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Calcarine sulcus Inferior occipital gyrus Lingual gyrus NP/MGH Central sulcus Superior parietal lobule Parietooccipital sulcus Frontopolar gyrus Frontomarginal gyrus Superior occipital gyrus Middle occipital gyrus Medial orbital gyrus Lingual gyrus Posterior orbital gyrus Inferior occipital gyrus Inferior temporal gyrus Temporal horn, lateral ventricle NP/MGH Central sulcus Superior Temporal gyrus Middle Temporal gyrus Inferior Temporal gyrus NP/MGH Central sulcus Superior parietal gyrus Inferior frontal gyrus Frontomarginal gyrus Anterior orbital gyrus Superior occipital gyrus Middle occipital Posterior orbital gyrus gyrus Superior Temporal gyrus Inferior -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

Connectivity Reveals Relationship of Brain Areas for Reward-Guided
Connectivity reveals relationship of brain areas for PNAS PLUS reward-guided learning and decision making in human and monkey frontal cortex Franz-Xaver Neuberta,1, Rogier B. Marsa,b,c, Jérôme Salleta, and Matthew F. S. Rushwortha,b aDepartment of Experimental Psychology, University of Oxford, Oxford OX1 3UD, United Kingdom and bCentre for Functional MRI of the Brain (FMRIB), Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, Oxford OX3 9DU, United Kingdom; and cDonders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, 6525 EZ Nijmegen, The Netherlands Edited by Ranulfo Romo, Universidad Nacional Autonóma de México, Mexico City, D.F., Mexico, and approved February 25, 2015 (received for review June 9, 2014) Reward-guided decision-making depends on a network of brain distinct processes for task control, error detection, and conflict regions. Among these are the orbitofrontal and the anterior resolution (13, 14). Reliable identification and location of ACC cingulate cortex. However, it is difficult to ascertain if these areas subcomponent regions could assist the resolution of such debates. constitute anatomical and functional unities, and how these areas In the present study we formally compared brain regions im- correspond between monkeys and humans. To address these plicated in reward-guided decision making and learning in humans questions we looked at connectivity profiles of these areas using and monkeys, and attempted to identify their key subdivisions in resting-state functional MRI in 38 humans and 25 macaque relation to function (Fig. 1). We used fMRI in 25 monkeys and 38 monkeys. We sought brain regions in the macaque that resembled humans to delineate the functional interactions of “decision- 10 human areas identified with decision making and brain regions making regions” with other areas in the brain while subjects were in the human that resembled six macaque areas identified with decision making.