PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10041.Full.Pdf
The Journal of Neuroscience, July 23, 2014 • 34(30):10041–10054 • 10041 Systems/Circuits Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders Sarah R. Heilbronner and Suzanne N. Haber Department of Pharmacology and Physiology, University of Rochester Medical Center, Rochester, New York 14642 The cingulum bundle (CB) is one of the brain’s major white matter pathways, linking regions associated with executive function, decision-making, and emotion. Neuroimaging has revealed that abnormalities in particular locations within the CB are associated with specific psychiatric disorders, including depression and bipolar disorder. However, the fibers using each portion of the CB remain unknown. In this study, we used anatomical tract-tracing in nonhuman primates (Macaca nemestrina, Macaca fascicularis, Macaca mulatta)toexaminetheorganizationofspecificcingulate,noncingulatefrontal,andsubcorticalpathwaysthroughtheCB.Thegoalswere as follows: (1) to determine connections that use the CB, (2) to establish through which parts of the CB these fibers travel, and (3) to relate the CB fiber pathways to the portions of the CB identified in humans as neurosurgical targets for amelioration of psychiatric disorders. Results indicate that cingulate, noncingulate frontal, and subcortical fibers all travel through the CB to reach both cingulate and noncin- gulate targets. However, many brain regions send projections through only part, not all, of the CB. For example, amygdala fibers are not present in the caudal portion of the dorsal CB. These results allow segmentation of the CB into four unique zones. We identify the specific connections that are abnormal in psychiatric disorders and affected by neurosurgical interventions, such as deep brain stimulation and cingulotomy. -
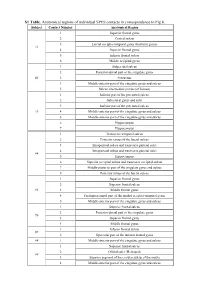
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Supporting Information for “Endocast Morphology of Homo Naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, S
Supporting Information for “Endocast Morphology of Homo naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, Shawn D. Hurst, Heather M. Garvin, P. Thomas Schoenemann, William B. Vanti, Lee R. Berger, and John Hawks What follows are our descriptions, illustrations, some basic interpretation, and a more specific discussion of the functional, comparative, and taxonomic issues surrounding these hominins. We use the neuroanatomical nomenclature from Duvernoy (19). DH1 Occipital The DH1 occipital fragment (Figs 1, S2) measures ca 105 mm in width between left temporo- occipital incisure and right sigmoid sinus. It is 61 mm in height on the left side, and 47 mm on the right side. The fragment covers the entire left and mostly complete right occipital lobes. The lobes are strongly asymmetrical, with the left clearly larger than the right, and more posteriorly protruding. There are faint traces of a lateral remnant of the lunate sulcus on the left side, and a dorsal bounding lunate as well (#4 and #6 in Fig 1). The right side shows a very small groove at the end of the lateral sinus, which could be a remnant of the lunate sulcus. The major flow from the longitudinal sinus is to the right. Small portions of both cerebellar lobes, roughly 15 mm in height are present. There is a suggestion of a great cerebellar sulcus on the right side. The width from the left lateral lunate impression to the midline is 43mm. The distance from left occipital pole (the most posteriorly projecting point, based on our best estimate of the proper orientation) to the mid-sagittal plane is 30 mm. -

On the Scent of Human Olfactory Orbitofrontal Cortex: Meta-Analysis and Comparison to Non-Human Primates
Brain Research Reviews 50 (2005) 287 – 304 www.elsevier.com/locate/brainresrev Review On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates Jay A. Gottfrieda,*, David H. Zaldb aDepartment of Neurology and the Cognitive Neurology and Alzheimer’s Disease Center, Northwestern University Feinberg School of Medicine, 320 E. Superior St., Searle 11-453, Chicago, IL 60611, USA bDepartment of Psychology, Vanderbilt University, Nashville, TN 37240, USA Accepted 25 August 2005 Available online 6 October 2005 Abstract It is widely accepted that the orbitofrontal cortex (OFC) represents the main neocortical target of primary olfactory cortex. In non-human primates, the olfactory neocortex is situated along the basal surface of the caudal frontal lobes, encompassing agranular and dysgranular OFC medially and agranular insula laterally, where this latter structure wraps onto the posterior orbital surface. Direct afferent inputs arrive from most primary olfactory areas, including piriform cortex, amygdala, and entorhinal cortex, in the absence of an obligatory thalamic relay. While such findings are almost exclusively derived from animal data, recent cytoarchitectonic studies indicate a close anatomical correspondence between non-human primate and human OFC. Given this cross-species conservation of structure, it has generally been presumed that the olfactory projection area in human OFC occupies the same posterior portions of OFC as seen in non-human primates. This review questions this assumption by providing a critical survey of the localization of primate and human olfactory neocortex. Based on a meta-analysis of human functional neuroimaging studies, the region of human OFC showing the greatest olfactory responsivity appears substantially rostral and in a different cytoarchitectural area than the orbital olfactory regions as defined in the monkey. -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -
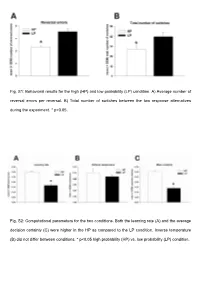
Fig. S1: Behavioral Results for the High (HP) and Low Probability (LP) Condition
Fig. S1: Behavioral results for the high (HP) and low probability (LP) condition. A) Average number of reversal errors per reversal. B) Total number of switches between the two response alternatives during the experiment. * p<0.05. Fig. S2: Computational parameters for the two conditions. Both the learning rate (A) and the average decision certainty (C) were higher in the HP as compared to the LP condition. Inverse temperature (B) did not differ between conditions. * p<0.05 high probability (HP) vs. low probability (LP) condition. Fig. S3: Signal change in response to negative feedback (ALLNEG – ALLPOS) superimposed on the MNI template brain. In both conditions (HP: top row, LP: bottom row), there was increased activity in the RCZ (left) and in the lateral prefrontal cortex (right). The color bar indicates z-scores. Tab. S1: Brain regions and MNI coordinates (x,y,z) of brain regions significantly activated in negative feedback trials that were followed by a behavioral adaptation after task rule reversal (FINREVERR – ALLPOS). Only clusters of at least 5 contiguous voxels are reported. L, left; R, right; BA, Brodmann area; RCZ, rostral cingulate zone. MNI coordinates Brain region z-score mm3 x y z HP condition L/R RCZ 4.79 7216 1 33 29 L inferior parietal lobule 4.58 7533 -52 -37 35 R inferior parietal lobule 4.6 8489 46 -51 35 R inferior/middle frontal gyrus 4.53 6346 34 18 28 L inferior frontal gyrus 4.1 318 -55 6 28 L pregenual BA 32 4.37 2080 -12 50 6 L middle frontal gyrus 4.49 1515 -41 42 27 R dorsal postcentral sulcus 4.46 1142 24 -40 59 L parieto-occipital transition cortex 4.09 146 -13 -70 33 L/R posterior cingulate cortex 4.1 1506 8 -17 30 paracentral lobule 3.57 323 -5 -39 63 LP condition L/R RCZ 4.48 3277 -2 36 28 R middle frontal gyrus 4.42 5057 46 38 26 L inferior frontal sulcus 4.16 613 -37 33 24 L/R ventral posterior cingulate cortex 4.38 770 -1 -24 27 L inferior frontal gyrus 3.93 371 -39 4 24 R inferior parietal lobule 4.02 362 43 -42 29 Tab. -

Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P
Cerebral Cortex, November 2015;25: 4351–4373 doi: 10.1093/cercor/bhv019 Advance Access Publication Date: 24 February 2015 Original Article ORIGINAL ARTICLE Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P. Aggleton1, Nicholas F. Wright1, Douglas L. Rosene2, and Richard C. Saunders3 1School of Psychology, Cardiff University, Cardiff, Wales CF10 3AT, UK, 2School of Medicine, Department of Anatomy and Neurobiology, Boston University, Boston MA 02118, USA, and 3Laboratory of Neuropsychology, National Institute of Mental Health, Bethesda, MD 20892, USA Address correspondence to: John P. Aggleton, School of Psychology, Cardiff University, Park Place, Cardiff, Wales CF10 3AT, UK. Email: [email protected] Abstract The projections from the amygdala and hippocampus (including subiculum and presubiculum) to prefrontal cortex were compared using anterograde tracers injected into macaque monkeys (Macaca fascicularis, Macaca mulatta). Almost all prefrontal areas were found to receive some amygdala inputs. These connections, which predominantly arose from the intermediate and magnocellular basal nucleus, were particularly dense in parts of the medial and orbital prefrontal cortex. Contralateral inputs were not, however, observed. The hippocampal projections to prefrontal areas were far more restricted, being confined to the ipsilateral medial and orbital prefrontal cortex (within areas 11, 13, 14, 24a, 32, and 25). These hippocampal projections principally arose from the subiculum, with the fornix providing the sole route. Thus, while the lateral prefrontal cortex essentially receives only amygdala inputs, the orbital prefrontal cortex receives both amygdala and hippocampal inputs, though these typically target different areas. Only in medial prefrontal cortex do direct inputs from both structures terminate in common sites. -

Jaw and Orofacial Motor Representation in Cat Orbital Gyrus 593
Jpn. J. Oral Biol., 33: 592-599, 1991. ORIGINAL Jaw and orofacial motor representation in cat orbital gyrus Yoshiyuki Tsuboi, Koichi Iwata, Hiroyuki Muramatsu, Junichi Yagi, Yuji Inomata, Reo Kikuta, Keiji Yoshii and Rhyuji Sumino Department of Physiology, Nihon University. School of Dentistry, 1-8-13 Kandasurugadai, Chiyoda-ku, Tokyo 101, Japan (Director: Prof. Rhyuji Sumino) Accepted for publication: June•k 20, 1991•l Key words: motor effect/trigeminal nerve/orbital gyrus/ICMS/cat Abstract: Jaw and orofacial motor representation in the orbital cortex was studied by intracortical microstimulation (ICMS) in lightly anesthetized cats. ICMS of the posterior portion of the the orbital gyrus produced movements of facial muscles, whereas stimulation of the anterior portion of the orbital gyrus produced more generalized movement of facial, jaw and tongue muscles. The region producing jaw movements was more restricted than the regions producing tongue and facial movements. Repe- titive stimulation of the anterior orbital gyrus produced either rhythmic jaw movements or sustained jaw opening. Cytoarchitectonically, the posterior portion of the orbital gyrus was restricted to area 43 and the anterior portion to areas 43 and 6 aƒÀ. present study, the function of the orbital gyrus Introduction was studied by detailed topographic mapping The orbital cortex in the cat has been ter- of the effects produced by ICMS and the med the cortical masticatory area because results correlated with the cytoarchitectonic criteria of Hassler and Muhs-Clement7). repetitive stimulation of its anterior part pro- duces rhythmic jaw and tongue move- Materials and Methods ments1-3). The axons of cells in this cortical region may project to a "pattern generator" Experiments were performed on 16 cats in the rhythmic generation of masticatory anesthetized initially with Ketamine HCL (50 jaw movements2,4). -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -

Not for Reprintlaboratory INVESTIGATION Anterior Peri-Insular Quadrantotomy: a Cadaveric White Matter Dissection Study
Authors: Please review all authors’ names, academicLow-resolution degrees, affiliations, and PDF contributions, as well as the Disclosure, MS# 19-472, read before authors for spelling and accuracy. Not for reprintLABORATORY INVESTIGATION Anterior peri-insular quadrantotomy: a cadaveric white matter dissection study *Pablo Gonzalez-Lopez, MD, PhD,1 Giulia Cossu, MD,2 Etienne Pralong, MD,2 Matias Baldoncini, MD,3 Mahmoud Messerer, MD, MSc,2,4 and Roy Thomas Daniel, MD, MCh2,4 1Department of Neurosurgery, Hospital General Universitario Alicante, Spain; 2Department of Neurosurgery, University Hospital of Lausanne, Switzerland; 3Department of Neurological Surgery, San Fernando Hospital, Buenos Aires, Argentina; and 4Faculty of Medicine and Biology, University of Lausanne, Switzerland OBJECTIVE Anterior quadrant disconnection represents a safe surgical option in well-selected pediatric patients with a large frontal lobe lesion anterior to the motor cortex. The understanding of the anatomy of the white matter tracts con- necting the frontal lobe with the rest of the cerebrum forms the basis of a safe and successful disconnective surgery. The authors explored and illustrated the relevant white matter tracts sectioned during each surgical step using fiber dissection techniques. METHODS Five human cadaveric hemispheres were dissected to illustrate the frontal connections in the 3 planes. The dissections were performed from lateral to medial, medial to lateral, and ventral to dorsal to describe the various tracts sectioned during the 4 steps of this surgery, namely the anterior suprainsular window, intrafrontal disconnection, anterior callosotomy, and frontobasal disconnection. RESULTS At the beginning of each surgical step, the U fibers were cut. During the anterior suprainsular window, the superior longitudinal fasciculus (SLF), the uncinate fasciculus, and the inferior fronto-occipital fasciculus (IFOF) were vi- sualized and sectioned, followed by sectioning of the anterior limb of the internal capsule.