Supplementary Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -
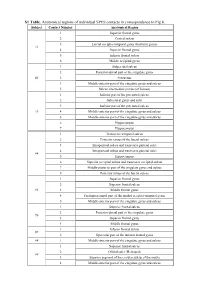
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Correction for Partial-Volume Effects on Brain Perfusion SPECT in Healthy Men
Correction for Partial-Volume Effects on Brain Perfusion SPECT in Healthy Men Hiroshi Matsuda, MD1; Takashi Ohnishi, MD1; Takashi Asada, MD2; Zhi-jie Li, MD1,3; Hidekazu Kanetaka, MD1; Etsuko Imabayashi, MD1; Fumiko Tanaka, MD1; and Seigo Nakano, MD4 1Department of Radiology, National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry, Tokyo, Japan; 2Department of Neuropsychiatry, Institute of Clinical Medicine, University of Tsukuba, Ibaraki, Japan; 3Department of Nuclear Medicine, The Second Clinical Hospital of China Medical University, Shen-Yang City, China; and 4Department of Geriatric Medicine, National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry, Tokyo, Japan The limited spatial resolution of SPECT scanners does not allow Functional changes in the brains of healthy elderly people an exact measurement of the local radiotracer concentration in and patients with neurodegenerative disorders have been brain tissue because partial-volume effects (PVEs) underesti- studied widely by SPECT. However, due to the limited mate concentration in small structures of the brain. The aim of this study was to determine which brain structures show greater spatial resolution of SPECT, the accurate measurement of influence of PVEs in SPECT studies on healthy volunteers and to tracer concentration in brain structures depends on several investigate aging effects on SPECT after the PVE correction. physical limitations—particularly, the relation between ob- Methods: Brain perfusion SPECT using 99mTc-ethylcysteinate ject size and scanner spatial resolution. This relation, known dimer was performed in 52 healthy men, 18–86 y old. The as the partial-volume effect (PVE), biases the measured regional cerebral blood flow (rCBF) was noninvasively measured concentration in small structures by diminishing the true using graphical analysis. -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Translingual Neural Stimulation with the Portable Neuromodulation
Translingual Neural Stimulation With the Portable Neuromodulation Stimulator (PoNS®) Induces Structural Changes Leading to Functional Recovery In Patients With Mild-To-Moderate Traumatic Brain Injury Authors: Jiancheng Hou,1 Arman Kulkarni,2 Neelima Tellapragada,1 Veena Nair,1 Yuri Danilov,3 Kurt Kaczmarek,3 Beth Meyerand,2 Mitchell Tyler,2,3 *Vivek Prabhakaran1 1. Department of Radiology, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin, USA 2. Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, Wisconsin, USA 3. Department of Kinesiology, University of Wisconsin-Madison, Madison, Wisconsin, USA *Correspondence to [email protected] Disclosure: Dr Tyler, Dr Danilov, and Dr Kaczmarek are co-founders of Advanced Neurorehabilitation, LLC, which holds the intellectual property rights to the PoNS® technology. Dr Tyler is a board member of NeuroHabilitation Corporation, a wholly- owned subsidiary of Helius Medical Technologies, and owns stock in the corporation. The other authors have declared no conflicts of interest. Acknowledgements: Professional medical writing and editorial assistance were provided by Kelly M. Fahrbach, Ashfield Healthcare Communications, part of UDG Healthcare plc, funded by Helius Medical Technologies. Dr Tyler, Dr Kaczmarek, Dr Danilov, Dr Hou, and Dr Prabhakaran were being supported by NHC-TBI-PoNS-RT001. Dr Hou, Dr Kulkarni, Dr Nair, Dr Tellapragada, and Dr Prabhakaran were being supported by R01AI138647. Dr Hou and Dr Prabhakaran were being supported by P01AI132132, R01NS105646. Dr Kulkarni was being supported by the Clinical & Translational Science Award programme of the National Center for Research Resources, NCATS grant 1UL1RR025011. Dr Meyerand, Dr Prabhakaran, Dr Nair was being supported by U01NS093650. -

Anatomic Significance of Topographical Relief in the Pars Basalis Telencephali
Rom J Leg Med [27] 1-9 [2019] DOI: 10.4323/rjlm.2019.1 © 2019 Romanian Society of Legal Medicine FUNDAMENTAL RESEARCH Anatomic significance of topographical relief in the pars basalis telencephali. Implications in forensic psychopathology Gheorghe S. Drăgoi1,2,*, Ileana Marinescu3 _________________________________________________________________________________________ Abstract: Topographical relief situated in the pars basalis telencephali have always been aspects of interest for anatomists and neurologists with regard to their terming and integration in neuronal systems. The main target of the present work is the macroanatonic analysis of the location and relations of reference anatomic markers of topographical relief on which connections of anatomical and functional areas are based. The study was carried out on human biological samples. Twenty samples of lesion- free encephala were taken from 8 men (aged between 36 and 65), 7 women (aged between 41 and 69) and 5 new-born infants (2 males and 3 females). Based on reference anatomic markers, identification of anatomic and functional area borderlines was done in the subcalloso – olfactory space. According to morphological variability the markers were grouped in 3 classes: a) commissural interconnective markers; b) non-commissural interconnective markers; c) markers modelled by the branches of the anterior cerebral artery. Based on our research and further corroboration with the progress made in the research of topographical relief as well as of the experimental results on septal nuclei, we offer an attempt at clarifying of the semantic content of taxonomy of terms as well as their implication in forensic phychopathology. Key Words: septum verum, subcallosal area, paraterminal gyrus, paraolfactory area, paraolfactory gyri. INTRODUCTION [7]; Cruveilhier (1836)[8]; Bergman (1831)[9]; Meynert (1867)[10]; Broca (1879)[11]; și Zuckerkandl (1887) Topographical relief variation in the pars basalis [12]. -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Differential Projection from the Motor and Limbic Cortical Regions to the Mediodorsal Thalamic Nucleus in the Dog
ACTA NEUROBIOL. EXP. 1989. 49: 23-37 DIFFERENTIAL PROJECTION FROM THE MOTOR AND LIMBIC CORTICAL REGIONS TO THE MEDIODORSAL THALAMIC NUCLEUS IN THE DOG Iwona STEPNIEWSKA and Anna KOSMAL Department of Neurophysiology, Nencki Institute of Experimental Biology 3 Pasteur Str., 02-093 Warsaw, Poland Key words: mediodorsal thalamic nucleus, motor and limbic cortices, horseradish peroxidase Abstract. The cortical afferents to the mediodorsal thalamic nucleus in the dog were studied by using horseradish peroxidase. Small injec- tions allowed to establish two specific projection zones connected sepa- rately with the lateral and medial segments of the nucleus. The lateral segment received the major projection from the dorsal half of the hemi- sphere. It included premotor and part of the motor cortices in the ante- rior sigmoid gyrus and precruciate areas as well as the presylvian cor- tex. The medial segment of the nucleus was innervated by the limbic areas of the ventral half of the hemisphere. These areas included the medloventrally located genual, subcallosal and plriform cortices, as well as the cortex of the ventral bank of the anterior rhinal sulcus and the caudal part of the orbital gyrus. The cortical fields situated between these two main cortical zones, both on the lateral and medial surfaces (rhinal and sylvian sulci and anterior cingular gyrus, respectively) sent projec- tions to both medial and lateral segments of the nucleus. These results indicate that in the mediodorsal thalamic nucleus may take place the integration of information from two functionally defined systems, the motor and limbic ones. INTRODUCTIOPJ The numerous studies of the cortical afferents of the mediodorsal thalamic nucleus (MD) showed, that although the prefrontal cortex (PFC) gives rise to the heaviest projection to MD, it is not the only cortical region projecting to thls nucleus. -

Dissection Technique for the Study of the Cerebral Sulci, Gyri and Ventricles
Arq Neuropsiquiatr 2008;66(2-A):282-287 Dissection technique for the stuDy of the cerebral sulci, gyri anD ventricles João Paulo Mattos1, Marcos Juliano dos Santos2, João Flavio Daniel Zullo2, Andrei Fernandes Joaquim2, Feres Chaddad-Neto1, Evandro de Oliveira3 Abstract – Neuroanatomy in addition to neurophysiology, are the basic areas for the proper formation from health students to specialized professionals in neuroscience. A step by step guide for practical studies of neuroanatomy is required for this kind of knowledge to become more acceptable among medical students, neurosurgeons, neurologists, neuropediatricians and psychiatric physicians. Based on the well known courses of sulci, gyri and ventricles offered by Beneficência Portuguesa Hospital in São Paulo, Brazil, two times a year, since 1994, totalizing more than 20 complete courses, and answering the request of many neuroscience students and professionals whose asked for a practical guide to the neuroanatomy study, the authors suggest a protocol for the study of superficial and deep brain structures showing how to approach the more structures as possible with minimum damage to the anatomic piece and with the smaller number of brains. Key wordS: neuroanatomy, brain, dissection technique. técnica de dissecação para o estudo dos sulcos, giros e ventriculos cerebrais Resumo – Neuroanatomia e a neurofisiologia são as áreas básicas para a adequada formação desde estudantes na área da saúde a profissionais especializados em neurociências. Um guia prático, passo a passo, para o estudo -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472