A Scoping Review of Neuromodulation Techniques in Neurodegenerative Diseases: a Useful Tool for Clinical Practice?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Cognitive Training for Older Adults: What Is It and Does It Work? Alexandra Kueider, Krystal Bichay, and George Rebok
Issue Brief OCTOBER 2014 Cognitive Training for Older Adults: What Is It and Does It Work? Alexandra Kueider, Krystal Bichay, and George Rebok Older adults are more likely to fear losing their mental abilities than their physical abilities.1 But a growing body of research suggests that, for most people, mental decline isn’t inevitable and may even be reversible. It is now becoming clear that cognitive health and dementia prevention must be lifelong pursuits, and the new approaches springing from a better understanding of the risk factors for cognitive impairment are far more promising than current drug therapies. Key points from our analysis of the current evidence What Is Known About Cognitive Training Now? include the following: Aggressive marketing notwithstanding, drugs mar- ■■ Cognitive training can improve cognitive abilities. keted for dementias such as AD do little to maintain Dementia drugs cannot. cognitive and functional abilities or slow the prog- ress of the disease. In contrast to drugs are mental ■■ No single cognitive training program stands out exercises to improve cognitive abilities. Dr. Richard as superior to others, but a group format based Suzman, Director of the Division of Behavioral and on multiple cognitive strategies seems the most Social Research at the National Institute on Aging promising. (NIA), states, “These sorts of interventions are poten- tially enormously important. The effects [of cogni- ■■ Research comparing cognitive exercise tive training interventions] were substantial. There approaches is still thin. Rigorous evaluation stan- isn’t a drug that will do that yet, and if there were, it dards are needed. would probably have to be administered with mental exercises.”2 ■■ Cognitive training could reduce health care costs by helping older individuals maintain a healthy Controversy and confusion still surround the efficacy and active lifestyle. -

Behavioral Neuroscience Uab Graduate Handbook
Behavioral Neuroscience Ph.D. Program Policies, Guidelines, & Procedures Student Handbook 2021-2022 University of Alabama at Birmingham Table of Contents Mission Statement __________________________________________ 3 History of the Program _______________________________________ 3 Policies and Procedures ______________________________________ 4 Overview of Student Career ___________________________________ 5 Typical Courses ____________________________________________ 5 Progress Reports ___________________________________________ 6 2nd Year Research Project __________________________________ 7 Qualifying Examination _______________________________________ 8 Dissertation ________________________________________________ 10 Behavioral Neuroscience Student Checklist _______________________ 13 Master’s Degree ____________________________________________ 15 Policies Regarding Adequate Progress __________________________ 16 Policies on Remunerated Activities _____________________________ 16 Vacation, Leave, Holiday Guidelines ____________________________ 17 Degree Requirements and Associated Procedures ________________ 18 2 BEHAVIORAL NEUROSCIENCE PROGRAM Mission Statement and History of the Program Mission Statement Behavioral neuroscience is represented by scientists with interests in the physiological and neural substrates of behavior. The mission of the Behavioral Neuroscience Ph.D. program is to produce outstanding young scientists capable of pursuing independent research careers in the field of behavioral neuroscience by providing graduate -

The Creation of Neuroscience
The Creation of Neuroscience The Society for Neuroscience and the Quest for Disciplinary Unity 1969-1995 Introduction rom the molecular biology of a single neuron to the breathtakingly complex circuitry of the entire human nervous system, our understanding of the brain and how it works has undergone radical F changes over the past century. These advances have brought us tantalizingly closer to genu- inely mechanistic and scientifically rigorous explanations of how the brain’s roughly 100 billion neurons, interacting through trillions of synaptic connections, function both as single units and as larger ensem- bles. The professional field of neuroscience, in keeping pace with these important scientific develop- ments, has dramatically reshaped the organization of biological sciences across the globe over the last 50 years. Much like physics during its dominant era in the 1950s and 1960s, neuroscience has become the leading scientific discipline with regard to funding, numbers of scientists, and numbers of trainees. Furthermore, neuroscience as fact, explanation, and myth has just as dramatically redrawn our cultural landscape and redefined how Western popular culture understands who we are as individuals. In the 1950s, especially in the United States, Freud and his successors stood at the center of all cultural expla- nations for psychological suffering. In the new millennium, we perceive such suffering as erupting no longer from a repressed unconscious but, instead, from a pathophysiology rooted in and caused by brain abnormalities and dysfunctions. Indeed, the normal as well as the pathological have become thoroughly neurobiological in the last several decades. In the process, entirely new vistas have opened up in fields ranging from neuroeconomics and neurophilosophy to consumer products, as exemplified by an entire line of soft drinks advertised as offering “neuro” benefits. -

The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’S Disease
life Review The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease Gianfranco Natale 1,2,* , Larisa Ryskalin 1 , Gabriele Morucci 1 , Gloria Lazzeri 1, Alessandro Frati 3,4 and Francesco Fornai 1,4 1 Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, 56126 Pisa, Italy; [email protected] (L.R.); [email protected] (G.M.); [email protected] (G.L.); [email protected] (F.F.) 2 Museum of Human Anatomy “Filippo Civinini”, University of Pisa, 56126 Pisa, Italy 3 Neurosurgery Division, Human Neurosciences Department, Sapienza University of Rome, 00135 Rome, Italy; [email protected] 4 Istituto di Ricovero e Cura a Carattere Scientifico (I.R.C.C.S.) Neuromed, 86077 Pozzilli, Italy * Correspondence: [email protected] Abstract: The gastrointestinal (GI) tract is provided with a peculiar nervous network, known as the enteric nervous system (ENS), which is dedicated to the fine control of digestive functions. This forms a complex network, which includes several types of neurons, as well as glial cells. Despite extensive studies, a comprehensive classification of these neurons is still lacking. The complexity of ENS is magnified by a multiple control of the central nervous system, and bidirectional communication between various central nervous areas and the gut occurs. This lends substance to the complexity of the microbiota–gut–brain axis, which represents the network governing homeostasis through nervous, endocrine, immune, and metabolic pathways. The present manuscript is dedicated to Citation: Natale, G.; Ryskalin, L.; identifying various neuronal cytotypes belonging to ENS in baseline conditions. -

Thinking About Actions: the Neural Substrates of Person Knowledge
Person Knowledge 1 Running Head: Person Knowledge Thinking About Actions: The Neural Substrates of Person Knowledge Malia F. Mason, Jane F. Banfield, and C. Neil Macrae Dartmouth College Address Correspondence To: Malia Mason Columbia Business School 720 Uris Hall New York, NY 10027 Email: [email protected] Phone: 212.854.1070 Person Knowledge 2 Abstract Despite an extensive literature on the neural substrates of semantic knowledge, how person-related information is represented in the brain has yet to be elucidated. Accordingly, in the present study we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of person knowledge. Focusing on the neural substrates of action knowledge, participants reported whether or not a common set of behaviors could be performed by people or dogs. While dogs and people are capable of performing many of the same actions (e.g., run, sit, bite), we surmised that the representation of this knowledge would be associated with distinct patterns of neural activity. Specifically, person judgments were expected to activate cortical areas associated with theory of mind (ToM) reasoning. The results supported this prediction. Whereas action-related judgments about dogs were associated with activity in various regions, including the occipital and parahippocampal gyri; identical judgments about people yielded activity in areas of prefrontal cortex, notably the right middle and medial frontal gyri. These findings suggest that person knowledge may be functionally dissociable from comparable information about other animals, with action-related judgments about people recruiting neural activity that is indicative of ToM reasoning. Key Words: Action Knowledge; fMRI; Social Cognition; Theory of Mind; Mentalizing. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

Prefrontal and Posterior Parietal Contributions to the Perceptual Awareness of Touch M
www.nature.com/scientificreports OPEN Prefrontal and posterior parietal contributions to the perceptual awareness of touch M. Rullmann1,2,5, S. Preusser1,5 & B. Pleger1,3,4* Which brain regions contribute to the perceptual awareness of touch remains largely unclear. We collected structural magnetic resonance imaging scans and neurological examination reports of 70 patients with brain injuries or stroke in S1 extending into adjacent parietal, temporal or pre-/frontal regions. We applied voxel-based lesion-symptom mapping to identify brain areas that overlap with an impaired touch perception (i.e., hypoesthesia). As expected, patients with hypoesthesia (n = 43) presented lesions in all Brodmann areas in S1 on postcentral gyrus (BA 1, 2, 3a, 3b). At the anterior border to BA 3b, we additionally identifed motor area BA 4p in association with hypoesthesia, as well as further ventrally the ventral premotor cortex (BA 6, BA 44), assumed to be involved in whole-body perception. At the posterior border to S1, we found hypoesthesia associated efects in attention-related areas such as the inferior parietal lobe and intraparietal sulcus. Downstream to S1, we replicated previously reported lesion-hypoesthesia associations in the parietal operculum and insular cortex (i.e., ventral pathway of somatosensory processing). The present fndings extend this pathway from S1 to the insular cortex by prefrontal and posterior parietal areas involved in multisensory integration and attention processes. Te primary somatosensory cortex (S1) in monkeys can be divided into four Brodmann areas: (BA) 1, 2, 3a, and 3b. Each BA consists of a somatotopically organized map that subserves distinct somatosensory functions1–3. -

Understanding Cognitive Skills and Brain Training Jennifer L
Understanding Cognitive Skills and Brain Training Jennifer L. Fisher, Ph.D., ABPP Reading Center, Rochester, MN November 14, 2012 Who am I (not)? ! Ph.D. in Child Psychology from Arizona State University; Board Certified through ABPP 2006 ! Fellowship at Mayo Clinic 1996-1998; Consultant 1998-2007; Supplemental Consultant 2008-present ! Specialty in Pediatric Psychology, treating children with physical illnesses (no neuropsychology specialty). ! Most important credential: Mother of two children, Mimi and Myles, one of whom has special learning issues Overview of Presentation ! Intelligence versus Achievement ! Cognitive skills – building blocks of achievement ! What is a learning disability? ! Using cognitive training to address LD: From Orton- Gillingham to online brain training ! Overview of current brain training efforts ! Buyer beware! Intelligence vs. Achievement ! Intelligence – ! Your capacity to learn, solve problems, think abstractly, adapt, manage complexity, structure your own behavior, etc. ! Measured by “IQ Tests” - Intelligence Quotient (e.g., WAIS-IV, WISC-IV, Cattell Culture Fair III, Woodcock-Johnson Tests of Cognitive Abilities-III, Stanford-Binet Intelligence Scales V) ! IQ once thought to be unitary construct; now best represented by multiple domains. For example, on the WISC IV ! Verbal Comprehension (VC) Index ! Perceptual Reasoning (PO) Index ! Processing Speed (PS) Index ! Working Memory (WM) Index ! IQ once thought to be stable; now we are coming to see that skills within IQ may be trained (e.g., Working Memory) Intelligence vs. Achievement ! Achievement ! “What a child has learned so far…” in various subjects ! Reading ! Math ! Written Language ! Assessment measures– Woodcock Johnson Tests of Achievement WJ-III, Wechsler Individual Achievement Test – WIAT, Wide Range Achievement Test (WRAT) ! IQ and Achievement generally correlated ! High IQ expect High Achievement ! Avg. -

Correction for Partial-Volume Effects on Brain Perfusion SPECT in Healthy Men
Correction for Partial-Volume Effects on Brain Perfusion SPECT in Healthy Men Hiroshi Matsuda, MD1; Takashi Ohnishi, MD1; Takashi Asada, MD2; Zhi-jie Li, MD1,3; Hidekazu Kanetaka, MD1; Etsuko Imabayashi, MD1; Fumiko Tanaka, MD1; and Seigo Nakano, MD4 1Department of Radiology, National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry, Tokyo, Japan; 2Department of Neuropsychiatry, Institute of Clinical Medicine, University of Tsukuba, Ibaraki, Japan; 3Department of Nuclear Medicine, The Second Clinical Hospital of China Medical University, Shen-Yang City, China; and 4Department of Geriatric Medicine, National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry, Tokyo, Japan The limited spatial resolution of SPECT scanners does not allow Functional changes in the brains of healthy elderly people an exact measurement of the local radiotracer concentration in and patients with neurodegenerative disorders have been brain tissue because partial-volume effects (PVEs) underesti- studied widely by SPECT. However, due to the limited mate concentration in small structures of the brain. The aim of this study was to determine which brain structures show greater spatial resolution of SPECT, the accurate measurement of influence of PVEs in SPECT studies on healthy volunteers and to tracer concentration in brain structures depends on several investigate aging effects on SPECT after the PVE correction. physical limitations—particularly, the relation between ob- Methods: Brain perfusion SPECT using 99mTc-ethylcysteinate ject size and scanner spatial resolution. This relation, known dimer was performed in 52 healthy men, 18–86 y old. The as the partial-volume effect (PVE), biases the measured regional cerebral blood flow (rCBF) was noninvasively measured concentration in small structures by diminishing the true using graphical analysis. -
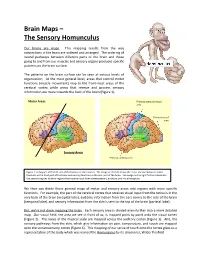
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Interference Resolution: Insights from a Meta-Analysis of Neuroimaging Tasks
Cognitive, Affective, & Behavioral Neuroscience 2007, 7 (1), 1-17 Interference resolution: Insights from a meta-analysis of neuroimaging tasks DEREK EVA N NEE University of Michigan, Ann Arbor, Michigan TOR D. WAGER Columbia University, New York, New York AND JOHN JONIDES University of Michigan, Ann Arbor, Michigan A quantitative meta-analysis was performed on 47 neuroimaging studies involving tasks purported to require the resolution of interference. The tasks included the Stroop, flanker, go/no-go, stimulus–response compatibil- ity, Simon, and stop signal tasks. Peak density-based analyses of these combined tasks reveal that the anterior cingulate cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, posterior parietal cortex, and anterior insula may be important sites for the detection and/or resolution of interference. Individual task analyses reveal differential patterns of activation among the tasks. We propose that the drawing of distinctions among the pro- cessing stages at which interference may be resolved may explain regional activation differences. Our analyses suggest that resolution processes acting upon stimulus encoding, response selection, and response execution may recruit different neural regions. The need to select information among competing al- shows the activations arising just from the Stroop task ternatives is ubiquitous. Oftentimes, successful cognition (Stroop, 1935), and these do not appear any more orderly. depends on the ability to focus resources on goal-relevant Indeed, the variability among the reported peaks across information while filtering out or inhibiting irrelevant infor- all interference resolution tasks corroborates behavioral mation. How selective attention operates and whether and findings that correlations in performance among different how irrelevant information is inhibited or otherwise filtered interference resolution tasks are low (Kramer, Humphrey, out has been a major focus of research since the inception Larish, Logan, & Strayer, 1994; Shilling, Chetwynd, & of experimental psychology.