Structure and Function of Visual Area MT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Visual Cortex in Humans 251
Author's personal copy Visual Cortex in Humans 251 Visual Cortex in Humans B A Wandell, S O Dumoulin, and A A Brewer, using fMRI, and we discuss the main features of the Stanford University, Stanford, CA, USA V1 map. We then summarize the positions and proper- ties of ten additional visual field maps. This represents ã 2009 Elsevier Ltd. All rights reserved. our current understanding of human visual field maps, although this remains an active field of investigation, with more maps likely to be discovered. Finally, we Human visua l cortex comprises 4–6 billion neurons that are organ ized into more than a dozen distinct describe theories about the functional purpose and functional areas. These areas include the gray matter organizing principles of these maps. in the occi pital lobe and extend into the temporal and parietal lobes . The locations of these areas in the The Size and Location of Human Visual intact human cortex can be identified by measuring Cortex visual field maps. The neurons within these areas have a variety of different stimulus response proper- The entirety of human cortex occupies a surface area 2 ties. We descr ibe how to measure these visual field on the order of 1000 cm and ranges between 2 and maps, their locations, and their overall organization. 4 mm in thickness. Each cubic millimeter of cortex contains approximately 50 000 neurons so that neo- We then consider how information about patterns, objects, color s, and motion is analyzed and repre- cortex in the two hemispheres contain on the order of sented in these maps. -
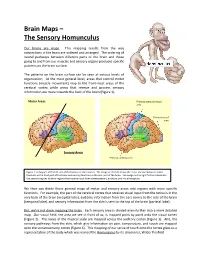
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Network Centrality in Patients with Acute Unilateral Open Globe Injury: a Voxel‑Wise Degree Centrality Study
MOLECULAR MEDICINE REPORTS 16: 8295-8300, 2017 Network centrality in patients with acute unilateral open globe injury: A voxel‑wise degree centrality study HUA WANG1, TING CHEN1, LEI YE2, QI-CHEN YANG3, RONG WEI2, YING ZHANG2, NAN JIANG2 and YI SHAO1,2 1Department of Ophthalmology, Xiangya Hospital, Central South University, Changsha, Hunan 410008; 2Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Jiangxi Province Clinical Ophthalmology Institute and Oculopathy Research Centre, Nanchang, Jiangxi 330006; 3Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Xiamen, Fujian 361102, P.R. China Received January 12, 2017; Accepted August 1, 2017 DOI: 10.3892/mmr.2017.7635 Abstract. The present study aimed to investigate functional Introduction networks underlying brain-activity alterations in patients with acute unilateral open globe injury (OGI) and associations with Open globe injury (OGI) is a severe eye disease that frequently their clinical features using the voxel-wise degree centrality causes unilateral visual loss. Ocular trauma is a public health (DC) method. In total, 18 patients with acute OGI (16 males and problem in developing countries (1,2). A previous study indicated 2 females), and 18 healthy subjects (16 males and 2 females), that the annual prevalence of ocular trauma was 4.9 per 100,000 closely matched in age, sex and education, participated in the in the Western Sicily Mediterranean area, which investigated a present study. Each subject underwent a resting-state functional 5 year period from January 2001 to December 2005 (3). In addi- magnetic resonance imaging scan. The DC method was used tion, the incidence of OGI is increased in men compared with to assess local features of spontaneous brain activity. -

Eye Fields in the Frontal Lobes of Primates
Brain Research Reviews 32Ž. 2000 413±448 www.elsevier.comrlocaterbres Full-length review Eye fields in the frontal lobes of primates Edward J. Tehovnik ), Marc A. Sommer, I-Han Chou, Warren M. Slocum, Peter H. Schiller Department of Brain and CognitiÕe Sciences, Massachusetts Institute of Technology, E25-634, Cambridge, MA 02139, USA Accepted 19 October 1999 Abstract Two eye fields have been identified in the frontal lobes of primates: one is situated dorsomedially within the frontal cortex and will be referred to as the eye field within the dorsomedial frontal cortexŽ. DMFC ; the other resides dorsolaterally within the frontal cortex and is commonly referred to as the frontal eye fieldŽ. FEF . This review documents the similarities and differences between these eye fields. Although the DMFC and FEF are both active during the execution of saccadic and smooth pursuit eye movements, the FEF is more dedicated to these functions. Lesions of DMFC minimally affect the production of most types of saccadic eye movements and have no effect on the execution of smooth pursuit eye movements. In contrast, lesions of the FEF produce deficits in generating saccades to briefly presented targets, in the production of saccades to two or more sequentially presented targets, in the selection of simultaneously presented targets, and in the execution of smooth pursuit eye movements. For the most part, these deficits are prevalent in both monkeys and humans. Single-unit recording experiments have shown that the DMFC contains neurons that mediate both limb and eye movements, whereas the FEF seems to be involved in the execution of eye movements only. -

The Human Brain Hemisphere Controls the Left Side of the Body and the Left What Makes the Human Brain Unique Is Its Size
About the brain Cerebrum (also known as the The brain is made up of around 100 billion nerve cells - each one cerebral cortex or forebrain) is connected to another 10,000. This means that, in total, we The cerebrum is the largest part of the brain. It is split in to two have around 1,000 trillion connections in our brains. (This would ‘halves’ of roughly equal size called hemispheres. The two be written as 1,000,000,000,000,000). These are ultimately hemispheres, the left and right, are joined together by a bundle responsible for who we are. Our brains control the decisions we of nerve fibres called the corpus callosum. The right make, the way we learn, move, and how we feel. The human brain hemisphere controls the left side of the body and the left What makes the human brain unique is its size. Our brains have a hemisphere controls the right side of the body. The cerebrum is larger cerebral cortex, or cerebrum, relative to the rest of the The human brain is the centre of our nervous further divided in to four lobes: frontal, parietal, occipital, and brain than any other animal. (See the Cerebrum section of this temporal, which have different functions. system. It is the most complex organ in our fact sheet for further information.) This enables us to have abilities The frontal lobe body and is responsible for everything we do - such as complex language, problem-solving and self-control. The frontal lobe is located at the front of the brain. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Study of Extra-Visual Resting-State Networks in Pituitary Adenoma Patients with Vision Restoration
Study of Extra-visual Resting-state Networks in Pituitary Adenoma Patients With Vision Restoration Fuyu Wang ( [email protected] ) Department of Neurosurgery, Chinese PLA General Hospital, Beijing, China https://orcid.org/0000- 0003-2232-5423 Peng Wang PLAGH: Chinese PLA General Hospital Ze Li PLAGH: Chinese PLA General Hospital Tao Zhou Chinese PLA General Hospital Xianghui Meng Chinese PLA General Hospital Jinli Jiang Chinese PLA General Hospital Research article Keywords: cross-modal plasticity, default mode network, resting-state functional magnetic resonance imaging, salience network, visual improvement Posted Date: December 29th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-133978/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/23 Abstract Background: Pituitary adenoma(PA) may compress the optic apparatus and cause impaired vision. Some patients can get improved vision rapidly after surgery. During the early time after surgery, however, the change of neurofunction in extra-visual cortex and higher cognitive cortex is still yet to be explored so far. Objective: Our study is focused on the changes in the extra-visual resting-state networks in PA patients after vision restoration. Methods:We recruited 14 PA patients with visual improvement after surgery. The functional connectivity (FC) of 6 seeds (auditory cortex (A1), Broca's area, posterior cingulate cortex (PCC)for default mode network (DMN), right caudal anterior cingulate cortex for salience network(SN) and left dorsolateral prefrontal cortex for excecutive control network (ECN)) were evaluated. A paired t-test was conducted to identify the differences between two groups. -

Neocortex: Consciousness Cerebellum
Grey matter (chips) White matter (the wiring: the brain mainly talks to itself) Neocortex: consciousness Cerebellum: unconscious control of posture & movement brains 1. Golgi-stained section of cerebral cortex 2. One of Ramon y Cajal’s faithful drawings showing nerve cell diversity in the brain cajal Neuropil: perhaps 1 km2 of plasma membrane - a molecular reaction substrate for 1024 voltage- and ligand-gated ion channels. light to Glia: 3 further cell types 1. Astrocytes: trophic interface with blood, maintain blood brain barrier, buffer excitotoxic neurotransmitters, support synapses astros Oligodendrocytes: myelin insulation oligos Production persists into adulthood: radiation myelopathy 3. Microglia: resident macrophages of the CNS. Similarities and differences with Langerhans cells, the professional antigen-presenting cells of the skin. 3% of all cells, normally renewed very slowly by division and immigration. Normal Neurosyphilis microglia Most adult neurons are already produced by birth Peak synaptic density by 3 months EMBRYONIC POSTNATAL week: 0 6 12 18 24 30 36 Month: 0 6 12 18 24 30 36 Year: 4 8 12 16 20 24 Cell birth Migration 2* Neurite outgrowth Synaptogenesis Myelination 1* Synapse elimination Modified from various sources inc: Andersen SL Neurosci & Biobehav Rev 2003 Rakic P Nat Rev Neurosci 2002 Bourgeois Acta Pediatr Suppl 422 1997 timeline 1 Synaptogenesis 100% * Rat RTH D BI E A Density of synapses in T PUBERTY primary visual cortex H at different times post- 0% conception. 100% (logarithmic scale) RTH Cat BI D E A T PUBERTY H The density values equivalent 0% to 100% vary between species 100% but in Man the peak value is Macaque 6 3 RTH 350 x10 synapses per mm BI D E PUBERTY A T The peak rate of synapse H formation is at birth in the 0% macaque: extrapolating to 100% the entire cortex, this Man RTH BI amounts to around 800,000 D E synapses formed per sec. -

Short- and Long-Term Deficits After Unilateral Ablation Andthe Effects of Subsequent Callosal Section’
0270.6474/84/0404-0918$02.00/0 The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 4, pp. 918-929 Printed in U.S.A. April 1984 SUPPLEMENTARY MOTOR AREA OF THE MONKEY’S CEREBRAL CORTEX: SHORT- AND LONG-TERM DEFICITS AFTER UNILATERAL ABLATION ANDTHE EFFECTS OF SUBSEQUENT CALLOSAL SECTION’ COBIE BRINKMAN Experimental Neurology Unit, The John Curtin School of Medical Research, Australian National University, Canberra, Australia 2601 Received March 7, 1983; Revised November 11, 1983; Accepted November 15, 1983 Abstract The short-term and long-term behavioral effects of unilateral lesions of the supplementary motor area (SMA) were studied in five monkeys (Mczcaca fascicularis ssp). A monkey with a unilateral lesion of the premotor area (PM) served as a control. In all animals, general behavior was unaffected by the lesions. For a few weeks postoperatively, all monkeys showed a clumsiness of forelimb movements, bilaterally, which involved both the distal and proximal muscles. Two SMA-lesioned monkeys (but not the PM-lesioned one), studied for up to 1 year postoperatively, showed a characteristic deficit of bimanual coordination where the two hands tended to behave in a similar manner instead of sharing the task between them. This deficit was more pronounced after a lesion contralateral to the nonpreferred hand. The deficit was interpreted as indicating that the intact SMA now influenced the motor outflow of both the ipsilateral hemisphere and the contralateral one through the corpus callosum. Callosal section immediately abolished the bimanual deficit, although the clumsiness returned transiently. The results imply that SMA may give rise normally to discharges informing the contralateral hemisphere of intended and/or ongoing movements via the corpus callosum. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Neurogenesis in Adult Mammals: Some Progress and Problems
The Journal of Neuroscience, February 1, 2002, 22(3):619–623 Neurogenesis in Adult Mammals: Some Progress and Problems Elizabeth Gould and Charles G. Gross Department of Psychology, Princeton University, Princeton, New Jersey 08544 Approximately 40 years after its first report, the addition of addition, the growing evidence that glia communicate among neurons to the brains of adult mammals has now been generally themselves electrically and have many functional properties that accepted. We now know that several endocrine and experiential interact and overlap with neurons (Rose and Konnerth, 2001; variables modulate adult neurogenesis. However, several meth- Bezzi and Volterra, 2001) suggests that new glia may play a odological problems in its quantitative study remain. One is the significant role. use of low doses of the exogenous marker of cell proliferation, In the 1990s there were several developments that finally es- bromodeoxyuridine (BrdU). A second is the transient lifetime of tablished neurogenesis in the adult rodent. The first was the most of the adult-generated cells. A third is that the survival of introduction of cell type-specific markers for immunohistochem- new neurons may depend on stimuli that are lacking in standard ical identification of the phenotype of newly generated cells laboratory conditions. This review considers these issues as well (Cameron et al., 1993; Okano et al., 1993; Seki and Arai, 1993). as the possible functions of new neurons. These could be combined with 3H-thymidine labeling to deter- From the beginnings of modern neuroscience in the late 19th mine the identity of the new cells. The second was the introduc- century, it was assumed that the mammalian CNS became struc- tion of the thymidine analog BrdU as another in vivo marker of turally stable soon after birth and remained that way throughout proliferating cells (Miller and Nowakowski, 1988; Seki and Arai life.