How Cells Fold the Cerebral Cortex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Brain Sulci and Gyri: a Practical Anatomical Review
Journal of Clinical Neuroscience 21 (2014) 2219–2225 Contents lists available at ScienceDirect Journal of Clinical Neuroscience journal homepage: www.elsevier.com/locate/jocn Neuroanatomical study Brain sulci and gyri: A practical anatomical review ⇑ Alvaro Campero a,b, , Pablo Ajler c, Juan Emmerich d, Ezequiel Goldschmidt c, Carolina Martins b, Albert Rhoton b a Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina b Department of Neurological Surgery, University of Florida, Gainesville, FL, USA c Department of Neurological Surgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina d Department of Anatomy, Universidad de la Plata, La Plata, Argentina article info abstract Article history: Despite technological advances, such as intraoperative MRI, intraoperative sensory and motor monitor- Received 26 December 2013 ing, and awake brain surgery, brain anatomy and its relationship with cranial landmarks still remains Accepted 23 February 2014 the basis of neurosurgery. Our objective is to describe the utility of anatomical knowledge of brain sulci and gyri in neurosurgery. This study was performed on 10 human adult cadaveric heads fixed in formalin and injected with colored silicone rubber. Additionally, using procedures done by the authors between Keywords: June 2006 and June 2011, we describe anatomical knowledge of brain sulci and gyri used to manage brain Anatomy lesions. Knowledge of the brain sulci and gyri can be used (a) to localize the craniotomy procedure, (b) to Brain recognize eloquent areas of the brain, and (c) to identify any given sulcus for access to deep areas of the Gyri Sulci brain. Despite technological advances, anatomical knowledge of brain sulci and gyri remains essential to Surgery perform brain surgery safely and effectively. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Introduction and Methods the Field of Neuroesthetics Is a Recent Marriage
Introduction and Methods The field of neuroesthetics is a recent marriage of the realms of neuroscience and art. The objective of neuroesthetics is to comprehend the perception and subjective experience of art in terms of their neural substrates. In this study, we examined the effect of a number of original pieces of art on the brain of the artist herself and on that of a novice as she experienced the artwork for the first time. This allowed us to compare neural responses not only amongst different visual conditions but also between an expert (i.e. the artist) and a novice. Lia Cook lent her artwork to be used in this study. The pieces, which she believes to have an innate emotional quality, are cotton and rayon textiles. All of the pieces are portraits with a somewhat abstract, pixelated appearance imparted by the medium. In order to better understand the neural effects of the woven facial images, we used several types of control images. These included (i) scrambled woven pieces, or textiles that were controlled for color, contrast, and size but contained no distinct facial forms; and (ii) photographs, which were all photographs of human faces but were printed on heavy paper and lacked the texture and unique visual appearance of the textiles. All of the pieces are 12.5 in. x 18 in. The expert and novice were each scanned with functional MRI while they viewed and touched the tapestries and photographs. The subjects completed 100 trials divided across two functional scans. Each trial lasted a total of 12 s, with jittered intervals of 4 s to 6 s between trials. -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -
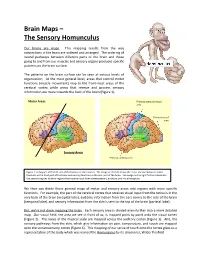
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

The Human Brain Hemisphere Controls the Left Side of the Body and the Left What Makes the Human Brain Unique Is Its Size
About the brain Cerebrum (also known as the The brain is made up of around 100 billion nerve cells - each one cerebral cortex or forebrain) is connected to another 10,000. This means that, in total, we The cerebrum is the largest part of the brain. It is split in to two have around 1,000 trillion connections in our brains. (This would ‘halves’ of roughly equal size called hemispheres. The two be written as 1,000,000,000,000,000). These are ultimately hemispheres, the left and right, are joined together by a bundle responsible for who we are. Our brains control the decisions we of nerve fibres called the corpus callosum. The right make, the way we learn, move, and how we feel. The human brain hemisphere controls the left side of the body and the left What makes the human brain unique is its size. Our brains have a hemisphere controls the right side of the body. The cerebrum is larger cerebral cortex, or cerebrum, relative to the rest of the The human brain is the centre of our nervous further divided in to four lobes: frontal, parietal, occipital, and brain than any other animal. (See the Cerebrum section of this temporal, which have different functions. system. It is the most complex organ in our fact sheet for further information.) This enables us to have abilities The frontal lobe body and is responsible for everything we do - such as complex language, problem-solving and self-control. The frontal lobe is located at the front of the brain. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Gliomas of the Cingulate Gyrus: Surgical Management and Functional Outcome
Neurosurg Focus 27 (2):E9, 2009 Gliomas of the cingulate gyrus: surgical management and functional outcome MAREC VON LEHE , M.D., AN D JOHANNES SCHRA mm , M.D. Neurochirurgische Klinik, Universitätsklinik Bonn, Germany Object. In this paper, the authors’ goal was to summarize their experience with the surgical treatment of gliomas arising from the cingulate gyrus. Methods. The authors analyzed preoperative data, surgical strategies, complications, and functional outcome in a series of 34 patients (mean age 42 years, range 12–69 years; 14 females) who underwent 38 operations between May 2001 and November 2008. Results. In 7 cases (18%) the tumor was located in the posterior (parietal) part of the cingulate gyrus, and in 31 (82%) the tumor was in the anterior (frontal) part. In 10 cases (26%) the glioma was solely located in the cingulate gyrus, and in 28 cases (74%) the tumor extended to the supracingular frontal/parietal cortex. Most cases (23 [61%]) had seizures as the presenting symptom, 8 patients (24%) suffered from a hemiparesis/hemihypesthesia, and 4 pa- tients (12%) had aphasic symptoms. The authors chose an interhemispheric approach for tumor resection in 11 (29%) and a transcortical approach in 27 (71%) cases; intraoperative electrophysiological monitoring was applied in 23 (61%) and neuronavigation in 15 (39%) cases. A > 90% resection was achieved in 32 (84%) and > 70% in another 5 (13%) cases. Tumors were classified as low-grade gliomas in 11 cases (29%). A glioblastoma multiforme (WHO Grade IV, 10 cases [26%]) and oligoastrocytoma (WHO Grade III, 9 cases [24%]) were the most frequent histopathological results. -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Neocortex: Consciousness Cerebellum
Grey matter (chips) White matter (the wiring: the brain mainly talks to itself) Neocortex: consciousness Cerebellum: unconscious control of posture & movement brains 1. Golgi-stained section of cerebral cortex 2. One of Ramon y Cajal’s faithful drawings showing nerve cell diversity in the brain cajal Neuropil: perhaps 1 km2 of plasma membrane - a molecular reaction substrate for 1024 voltage- and ligand-gated ion channels. light to Glia: 3 further cell types 1. Astrocytes: trophic interface with blood, maintain blood brain barrier, buffer excitotoxic neurotransmitters, support synapses astros Oligodendrocytes: myelin insulation oligos Production persists into adulthood: radiation myelopathy 3. Microglia: resident macrophages of the CNS. Similarities and differences with Langerhans cells, the professional antigen-presenting cells of the skin. 3% of all cells, normally renewed very slowly by division and immigration. Normal Neurosyphilis microglia Most adult neurons are already produced by birth Peak synaptic density by 3 months EMBRYONIC POSTNATAL week: 0 6 12 18 24 30 36 Month: 0 6 12 18 24 30 36 Year: 4 8 12 16 20 24 Cell birth Migration 2* Neurite outgrowth Synaptogenesis Myelination 1* Synapse elimination Modified from various sources inc: Andersen SL Neurosci & Biobehav Rev 2003 Rakic P Nat Rev Neurosci 2002 Bourgeois Acta Pediatr Suppl 422 1997 timeline 1 Synaptogenesis 100% * Rat RTH D BI E A Density of synapses in T PUBERTY primary visual cortex H at different times post- 0% conception. 100% (logarithmic scale) RTH Cat BI D E A T PUBERTY H The density values equivalent 0% to 100% vary between species 100% but in Man the peak value is Macaque 6 3 RTH 350 x10 synapses per mm BI D E PUBERTY A T The peak rate of synapse H formation is at birth in the 0% macaque: extrapolating to 100% the entire cortex, this Man RTH BI amounts to around 800,000 D E synapses formed per sec.