Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thinking About Actions: the Neural Substrates of Person Knowledge
Person Knowledge 1 Running Head: Person Knowledge Thinking About Actions: The Neural Substrates of Person Knowledge Malia F. Mason, Jane F. Banfield, and C. Neil Macrae Dartmouth College Address Correspondence To: Malia Mason Columbia Business School 720 Uris Hall New York, NY 10027 Email: [email protected] Phone: 212.854.1070 Person Knowledge 2 Abstract Despite an extensive literature on the neural substrates of semantic knowledge, how person-related information is represented in the brain has yet to be elucidated. Accordingly, in the present study we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of person knowledge. Focusing on the neural substrates of action knowledge, participants reported whether or not a common set of behaviors could be performed by people or dogs. While dogs and people are capable of performing many of the same actions (e.g., run, sit, bite), we surmised that the representation of this knowledge would be associated with distinct patterns of neural activity. Specifically, person judgments were expected to activate cortical areas associated with theory of mind (ToM) reasoning. The results supported this prediction. Whereas action-related judgments about dogs were associated with activity in various regions, including the occipital and parahippocampal gyri; identical judgments about people yielded activity in areas of prefrontal cortex, notably the right middle and medial frontal gyri. These findings suggest that person knowledge may be functionally dissociable from comparable information about other animals, with action-related judgments about people recruiting neural activity that is indicative of ToM reasoning. Key Words: Action Knowledge; fMRI; Social Cognition; Theory of Mind; Mentalizing. -

Brain Sulci and Gyri: a Practical Anatomical Review
Journal of Clinical Neuroscience 21 (2014) 2219–2225 Contents lists available at ScienceDirect Journal of Clinical Neuroscience journal homepage: www.elsevier.com/locate/jocn Neuroanatomical study Brain sulci and gyri: A practical anatomical review ⇑ Alvaro Campero a,b, , Pablo Ajler c, Juan Emmerich d, Ezequiel Goldschmidt c, Carolina Martins b, Albert Rhoton b a Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina b Department of Neurological Surgery, University of Florida, Gainesville, FL, USA c Department of Neurological Surgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina d Department of Anatomy, Universidad de la Plata, La Plata, Argentina article info abstract Article history: Despite technological advances, such as intraoperative MRI, intraoperative sensory and motor monitor- Received 26 December 2013 ing, and awake brain surgery, brain anatomy and its relationship with cranial landmarks still remains Accepted 23 February 2014 the basis of neurosurgery. Our objective is to describe the utility of anatomical knowledge of brain sulci and gyri in neurosurgery. This study was performed on 10 human adult cadaveric heads fixed in formalin and injected with colored silicone rubber. Additionally, using procedures done by the authors between Keywords: June 2006 and June 2011, we describe anatomical knowledge of brain sulci and gyri used to manage brain Anatomy lesions. Knowledge of the brain sulci and gyri can be used (a) to localize the craniotomy procedure, (b) to Brain recognize eloquent areas of the brain, and (c) to identify any given sulcus for access to deep areas of the Gyri Sulci brain. Despite technological advances, anatomical knowledge of brain sulci and gyri remains essential to Surgery perform brain surgery safely and effectively. -
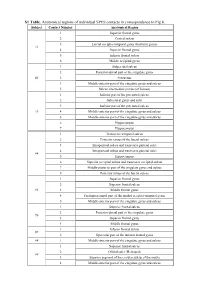
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Connectivity of BA46 Involvement in the Executive Control of Language
Alfredo Ardila, Byron Bernal and Monica Rosselli Psicothema 2016, Vol. 28, No. 1, 26-31 ISSN 0214 - 9915 CODEN PSOTEG Copyright © 2016 Psicothema doi: 10.7334/psicothema2015.174 www.psicothema.com Connectivity of BA46 involvement in the executive control of language Alfredo Ardila1, Byron Bernal2 and Monica Rosselli3 1 Florida International University, 2 Miami Children’s Hospital and 3 Florida Atlantic University Abstract Resumen Background: Understanding the functions of different brain areas has Estudio de la conectividad del AB46 en el control ejecutivo del lenguaje. represented a major endeavor of contemporary neurosciences. Modern Antecedentes: la comprensión de las funciones de diferentes áreas neuroimaging developments suggest cognitive functions are associated cerebrales representa una de las mayores empresas de las neurociencias with networks rather than with specifi c areas. Objectives. The purpose contemporáneas. Los estudios contemporáneos con neuroimágenes of this paper was to analyze the connectivity of Brodmann area (BA) 46 sugieren que las funciones cognitivas se asocian con redes más que con (anterior middle frontal gyrus) in relation to language. Methods: A meta- áreas específi cas. El propósito de este estudio fue analizar la conectividad analysis was conducted to assess the language network in which BA46 is del área de Brodmann 46 (BA46) (circunvolución frontal media anterior) involved. The DataBase of Brainmap was used; 19 papers corresponding con relación al lenguaje. Método: se llevó a cabo un meta-análisis para to 60 experimental conditions with a total of 245 subjects were included. determinar el circuito o red lingüística en la cual participa BA46. Se utilizó Results: Our results suggest the core network of BA46. -

Dorsolateral Prefrontal Cortex-Based Control with an Implanted Brain–Computer Interface
www.nature.com/scientificreports OPEN Dorsolateral prefrontal cortex‑based control with an implanted brain–computer interface Sacha Leinders 1, Mariska J. Vansteensel 1, Mariana P. Branco 1, Zac V. Freudenburg1, Elmar G. M. Pels1, Benny Van der Vijgh1, Martine J. E. Van Zandvoort2, Nicolas F. Ramsey1* & Erik J. Aarnoutse 1 The objective of this study was to test the feasibility of using the dorsolateral prefrontal cortex as a signal source for brain–computer interface control in people with severe motor impairment. We implanted two individuals with locked‑in syndrome with a chronic brain–computer interface designed to restore independent communication. The implanted system (Utrecht NeuroProsthesis) included electrode strips placed subdurally over the dorsolateral prefrontal cortex. In both participants, counting backwards activated the dorsolateral prefrontal cortex consistently over the course of 47 and 22months, respectively. Moreover, both participants were able to use this signal to control a cursor in one dimension, with average accuracy scores of 78 ± 9% (standard deviation) and 71 ± 11% (chance level: 50%), respectively. Brain– computer interface control based on dorsolateral prefrontal cortex activity is feasible in people with locked‑in syndrome and may become of relevance for those unable to use sensorimotor signals for control. Locked-in syndrome (LIS) is characterized by intact cognition and (almost) complete loss of voluntary movement1. Brain–computer interface (BCI) systems allow computer control with brain activity and can there- fore provide an alternative communication channel to people with LIS. BCI systems depending on self-initiated modulation of brain activity generally measure from sensorimotor areas, e.g.2–9. However, not all people with LIS may be able to reliably modulate sensorimotor activity, due to for instance atrophy secondary to neurode- generative disease10,11, damage afer stroke or injury, or atypical neural activity 12. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

The Role of the Superior Temporal Sulcus and the Mirror Neuron System in Imitation
r Human Brain Mapping 31:1316–1326 (2010) r The Role of the Superior Temporal Sulcus and the Mirror Neuron System in Imitation Pascal Molenberghs,* Christopher Brander, Jason B. Mattingley, and Ross Cunnington The University of Queensland, Queensland Brain Institute & School of Psychology, St Lucia, Queensland, Australia r r Abstract: It has been suggested that in humans the mirror neuron system provides a neural substrate for imitation behaviour, but the relative contributions of different brain regions to the imitation of manual actions is still a matter of debate. To investigate the role of the mirror neuron system in imita- tion we used fMRI to examine patterns of neural activity under four different conditions: passive ob- servation of a pantomimed action (e.g., hammering a nail); (2) imitation of an observed action; (3) execution of an action in response to a word cue; and (4) self-selected execution of an action. A net- work of cortical areas, including the left supramarginal gyrus, left superior parietal lobule, left dorsal premotor area and bilateral superior temporal sulcus (STS), was significantly active across all four con- ditions. Crucially, within this network the STS bilaterally was the only region in which activity was significantly greater for action imitation than for the passive observation and execution conditions. We suggest that the role of the STS in imitation is not merely to passively register observed biological motion, but rather to actively represent visuomotor correspondences between one’s own actions and the actions of others. Hum Brain Mapp 31:1316–1326, 2010. VC 2010 Wiley-Liss, Inc. Key words: fMRI; imitation; mirror neuron system r r INTRODUCTION ror neurons are visuomotor neurons that fire both when an action is performed and when a similar or identical Motor imitation involves observing the action of another action is passively observed [Rizzolatti and Craighero, individual and matching one’s own movements to those 2004]. -

Introduction and Methods the Field of Neuroesthetics Is a Recent Marriage
Introduction and Methods The field of neuroesthetics is a recent marriage of the realms of neuroscience and art. The objective of neuroesthetics is to comprehend the perception and subjective experience of art in terms of their neural substrates. In this study, we examined the effect of a number of original pieces of art on the brain of the artist herself and on that of a novice as she experienced the artwork for the first time. This allowed us to compare neural responses not only amongst different visual conditions but also between an expert (i.e. the artist) and a novice. Lia Cook lent her artwork to be used in this study. The pieces, which she believes to have an innate emotional quality, are cotton and rayon textiles. All of the pieces are portraits with a somewhat abstract, pixelated appearance imparted by the medium. In order to better understand the neural effects of the woven facial images, we used several types of control images. These included (i) scrambled woven pieces, or textiles that were controlled for color, contrast, and size but contained no distinct facial forms; and (ii) photographs, which were all photographs of human faces but were printed on heavy paper and lacked the texture and unique visual appearance of the textiles. All of the pieces are 12.5 in. x 18 in. The expert and novice were each scanned with functional MRI while they viewed and touched the tapestries and photographs. The subjects completed 100 trials divided across two functional scans. Each trial lasted a total of 12 s, with jittered intervals of 4 s to 6 s between trials. -

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Interference Resolution: Insights from a Meta-Analysis of Neuroimaging Tasks
Cognitive, Affective, & Behavioral Neuroscience 2007, 7 (1), 1-17 Interference resolution: Insights from a meta-analysis of neuroimaging tasks DEREK EVA N NEE University of Michigan, Ann Arbor, Michigan TOR D. WAGER Columbia University, New York, New York AND JOHN JONIDES University of Michigan, Ann Arbor, Michigan A quantitative meta-analysis was performed on 47 neuroimaging studies involving tasks purported to require the resolution of interference. The tasks included the Stroop, flanker, go/no-go, stimulus–response compatibil- ity, Simon, and stop signal tasks. Peak density-based analyses of these combined tasks reveal that the anterior cingulate cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, posterior parietal cortex, and anterior insula may be important sites for the detection and/or resolution of interference. Individual task analyses reveal differential patterns of activation among the tasks. We propose that the drawing of distinctions among the pro- cessing stages at which interference may be resolved may explain regional activation differences. Our analyses suggest that resolution processes acting upon stimulus encoding, response selection, and response execution may recruit different neural regions. The need to select information among competing al- shows the activations arising just from the Stroop task ternatives is ubiquitous. Oftentimes, successful cognition (Stroop, 1935), and these do not appear any more orderly. depends on the ability to focus resources on goal-relevant Indeed, the variability among the reported peaks across information while filtering out or inhibiting irrelevant infor- all interference resolution tasks corroborates behavioral mation. How selective attention operates and whether and findings that correlations in performance among different how irrelevant information is inhibited or otherwise filtered interference resolution tasks are low (Kramer, Humphrey, out has been a major focus of research since the inception Larish, Logan, & Strayer, 1994; Shilling, Chetwynd, & of experimental psychology. -

Reduced Amplitude of Low-Frequency Brain Oscillations in the Psychosis Risk Syndrome and Early Illness Schizophrenia
Neuropsychopharmacology (2016) 41, 2388–2398 © 2016 American College of Neuropsychopharmacology. All rights reserved 0893-133X/16 www.neuropsychopharmacology.org Reduced Amplitude of Low-Frequency Brain Oscillations in the Psychosis Risk Syndrome and Early Illness Schizophrenia 1,2 2 2 1 1,2 Susanna L Fryer , Brian J Roach , Katherine Wiley , Rachel L Loewy , Judy M Ford and ,1,2 Daniel H Mathalon* 1 2 Department of Psychiatry, University of California, San Francisco, San Francisco, CA, USA; San Francisco VA Medical Center, San Francisco, CA, USA Low-frequency oscillations (LFOs) of the blood oxygen level-dependent (BOLD) signal are gaining interest as potential biomarkers sensitive to neuropsychiatric pathology. Schizophrenia has been associated with alterations in intrinsic LFOs that covary with cognitive deficits and symptoms. However, the extent to which LFO dysfunction is present before schizophrenia illness onset remains unknown. = = Resting-state FMRI data were collected from clinical high-risk (CHR; n 45) youth, early illness schizophrenia (ESZ; n 74) patients, and = – healthy controls (HCs; n 85) aged 12 35 years. Age-adjusted voxelwise fractional amplitude of low-frequency fluctuations (fALFF; 0.01 − 0.08 Hz) of the BOLD signal was compared among the three groups. Main effects of Group (po0.005 height threshold, familywise error cluster-level corrected po0.05) were followed up via Tukey-corrected pairwise comparisons. Significant main effects of Group (po0.05) revealed decreased fALFF in ESZ and CHR groups relative to HCs, with values in the CHR group falling between those of ESZ and HC groups. These differences were identified primarily in posterior cortex, including temporoparietal regions, extending into occipital and cerebellar lobes.