Short- and Long-Term Deficits After Unilateral Ablation Andthe Effects of Subsequent Callosal Section’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -
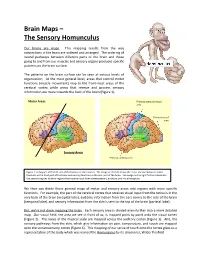
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Functional Role of the Supplementary and Pre-Supplementary Motor Areas
REVIEWS Functional role of the supplementary and pre-supplementary motor areas Parashkev Nachev*‡, Christopher Kennard‡ and Masud Husain* Abstract | The supplementary motor complex consists of the supplementary motor area, the supplementary eye field and the pre-supplementary motor area. In recent years, these areas have come under increasing scrutiny from cognitive neuroscientists, motor physiologists and clinicians because they seem to be crucial for linking cognition to action. However, theories regarding their function vary widely. This Review brings together the data regarding the supplementary motor regions, highlighting outstanding issues and providing new perspectives for understanding their functions. Ever since they were first identified, the regions that which we hope will stimulate the development of comprise the supplementary motor complex (SMC) have conceptual frameworks for a better understanding remained, in large part, a mystery. Most investigators now of this region. appreciate that these brain regions are far from ‘supple- mentary’ to requirements1–7. Without them, there are Anatomy and connections profound alterations in behaviour. For example, lesions to The supplementary motor area (SMA) and pre- these areas in humans can lead to alien-limb syndrome, supplementary motor area (pre-SMA) are, in humans, with patients demonstrating involuntary actions such as located on the medial aspect of the brain: in the dorso- grasping nearby objects — even other people — without medial frontal cortex3,14, anterior to the leg representa- ever intending to do so8,9. Some individuals demonstrate tion of the primary motor cortex (FIG. 1). Both areas lie utilization behaviour: unable to resist the impulse to use in the superior frontal gyrus and constitute the medial an object that has been placed within their reach, even part of Brodmann’s area 6c (later divided into two areas: when the object is not needed10. -

The Human Brain Hemisphere Controls the Left Side of the Body and the Left What Makes the Human Brain Unique Is Its Size
About the brain Cerebrum (also known as the The brain is made up of around 100 billion nerve cells - each one cerebral cortex or forebrain) is connected to another 10,000. This means that, in total, we The cerebrum is the largest part of the brain. It is split in to two have around 1,000 trillion connections in our brains. (This would ‘halves’ of roughly equal size called hemispheres. The two be written as 1,000,000,000,000,000). These are ultimately hemispheres, the left and right, are joined together by a bundle responsible for who we are. Our brains control the decisions we of nerve fibres called the corpus callosum. The right make, the way we learn, move, and how we feel. The human brain hemisphere controls the left side of the body and the left What makes the human brain unique is its size. Our brains have a hemisphere controls the right side of the body. The cerebrum is larger cerebral cortex, or cerebrum, relative to the rest of the The human brain is the centre of our nervous further divided in to four lobes: frontal, parietal, occipital, and brain than any other animal. (See the Cerebrum section of this temporal, which have different functions. system. It is the most complex organ in our fact sheet for further information.) This enables us to have abilities The frontal lobe body and is responsible for everything we do - such as complex language, problem-solving and self-control. The frontal lobe is located at the front of the brain. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Neocortex: Consciousness Cerebellum
Grey matter (chips) White matter (the wiring: the brain mainly talks to itself) Neocortex: consciousness Cerebellum: unconscious control of posture & movement brains 1. Golgi-stained section of cerebral cortex 2. One of Ramon y Cajal’s faithful drawings showing nerve cell diversity in the brain cajal Neuropil: perhaps 1 km2 of plasma membrane - a molecular reaction substrate for 1024 voltage- and ligand-gated ion channels. light to Glia: 3 further cell types 1. Astrocytes: trophic interface with blood, maintain blood brain barrier, buffer excitotoxic neurotransmitters, support synapses astros Oligodendrocytes: myelin insulation oligos Production persists into adulthood: radiation myelopathy 3. Microglia: resident macrophages of the CNS. Similarities and differences with Langerhans cells, the professional antigen-presenting cells of the skin. 3% of all cells, normally renewed very slowly by division and immigration. Normal Neurosyphilis microglia Most adult neurons are already produced by birth Peak synaptic density by 3 months EMBRYONIC POSTNATAL week: 0 6 12 18 24 30 36 Month: 0 6 12 18 24 30 36 Year: 4 8 12 16 20 24 Cell birth Migration 2* Neurite outgrowth Synaptogenesis Myelination 1* Synapse elimination Modified from various sources inc: Andersen SL Neurosci & Biobehav Rev 2003 Rakic P Nat Rev Neurosci 2002 Bourgeois Acta Pediatr Suppl 422 1997 timeline 1 Synaptogenesis 100% * Rat RTH D BI E A Density of synapses in T PUBERTY primary visual cortex H at different times post- 0% conception. 100% (logarithmic scale) RTH Cat BI D E A T PUBERTY H The density values equivalent 0% to 100% vary between species 100% but in Man the peak value is Macaque 6 3 RTH 350 x10 synapses per mm BI D E PUBERTY A T The peak rate of synapse H formation is at birth in the 0% macaque: extrapolating to 100% the entire cortex, this Man RTH BI amounts to around 800,000 D E synapses formed per sec. -

Down but Not out in Posterior Cingulate Cortex: Deactivation Yet Functional Coupling with Prefrontal Cortex During Demanding Semantic Cognition
NeuroImage 141 (2016) 366–377 Contents lists available at ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Down but not out in posterior cingulate cortex: Deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition Katya Krieger-Redwood ⁎, Elizabeth Jefferies, Theodoros Karapanagiotidis, Robert Seymour, Adonany Nunes, Jit Wei Aaron Ang, Vierra Majernikova, Giovanna Mollo, Jonathan Smallwood Department of Psychology/York Neuroimaging Centre, University of York, Heslington, York, United Kingdom article info abstract Article history: The posterior cingulate cortex (pCC) often deactivates during complex tasks, and at rest is often only weakly cor- Received 30 March 2016 related with regions that play a general role in the control of cognition. These observations led to the hypothesis Accepted 29 July 2016 that pCC contributes to automatic aspects of memory retrieval and cognition. Recent work, however, has sug- Available online 30 July 2016 gested that the pCC may support both automatic and controlled forms of memory processing and may do so by changing its communication with regions that are important in the control of cognition across multiple do- Keywords: mains. The current study examined these alternative views by characterising the functional coupling of the Posterior cingulate cortex Dorsolateral prefrontal cortex pCC in easy semantic decisions (based on strong global associations) and in harder semantic tasks (matching Semantic control words on the basis of specific non-dominant features). Increasingly difficult semantic decisions led to the expect- Executive ed pattern of deactivation in the pCC; however, psychophysiological interaction analysis revealed that, under Rest these conditions, the pCC exhibited greater connectivity with dorsolateral prefrontal cortex (PFC), relative to Connectivity both easier semantic decisions and to a period of rest. -

Neurogenesis in Adult Mammals: Some Progress and Problems
The Journal of Neuroscience, February 1, 2002, 22(3):619–623 Neurogenesis in Adult Mammals: Some Progress and Problems Elizabeth Gould and Charles G. Gross Department of Psychology, Princeton University, Princeton, New Jersey 08544 Approximately 40 years after its first report, the addition of addition, the growing evidence that glia communicate among neurons to the brains of adult mammals has now been generally themselves electrically and have many functional properties that accepted. We now know that several endocrine and experiential interact and overlap with neurons (Rose and Konnerth, 2001; variables modulate adult neurogenesis. However, several meth- Bezzi and Volterra, 2001) suggests that new glia may play a odological problems in its quantitative study remain. One is the significant role. use of low doses of the exogenous marker of cell proliferation, In the 1990s there were several developments that finally es- bromodeoxyuridine (BrdU). A second is the transient lifetime of tablished neurogenesis in the adult rodent. The first was the most of the adult-generated cells. A third is that the survival of introduction of cell type-specific markers for immunohistochem- new neurons may depend on stimuli that are lacking in standard ical identification of the phenotype of newly generated cells laboratory conditions. This review considers these issues as well (Cameron et al., 1993; Okano et al., 1993; Seki and Arai, 1993). as the possible functions of new neurons. These could be combined with 3H-thymidine labeling to deter- From the beginnings of modern neuroscience in the late 19th mine the identity of the new cells. The second was the introduc- century, it was assumed that the mammalian CNS became struc- tion of the thymidine analog BrdU as another in vivo marker of turally stable soon after birth and remained that way throughout proliferating cells (Miller and Nowakowski, 1988; Seki and Arai life. -

How Cells Fold the Cerebral Cortex
776 • The Journal of Neuroscience, January 24, 2018 • 38(4):776–783 Dual Perspectives Dual Perspectives Companion Paper: How Forces Fold the Cerebral Cortex, by Christopher D. Kroenke and Philip V. Bayly How Cells Fold the Cerebral Cortex Víctor Borrell Instituto de Neurociencias, Consejo Superior de Investigaciones Científicas & Universidad Miguel Herna´ndez, Sant Joan d’Alacant 03550, Spain Foldingofthecerebralcortexisashighlyintriguingaspoorlyunderstood.Atfirstsight,thismayappearassimpletissuecrumplinginside an excessively small cranium, but the process is clearly much more complex and developmentally predetermined. Whereas theoretical modeling supports a critical role for biomechanics, experimental evidence demonstrates the fundamental role of specific progenitor cell types, cellular processes, and genetic programs on cortical folding. Key words: basal Radial Glia; ferret; neurogenesis; OSVZ; Pax6; primate Introduction impact in defining the prospective patterns of cortical folding. In One of the most characteristic features of the human brain is its order for me to review the cellular mechanisms responsible for external folded appearance, due to the 3D arrangement of the cortical folding, I will first begin by defining what is cortical fold- cerebral cortex mantle forming outward folds and inward fis- ing, and what is not. sures. Folding patterns vary significantly between species, while being stereotyped within species. Developmental mechanisms re- Cortical folding sponsible for cortical folding are an intriguing and elusive ques- -

A Practical Review of Functional MRI Anatomy of the Language and Motor Systems
REVIEW ARTICLE FUNCTIONAL A Practical Review of Functional MRI Anatomy of the Language and Motor Systems X V.B. Hill, X C.Z. Cankurtaran, X B.P. Liu, X T.A. Hijaz, X M. Naidich, X A.J. Nemeth, X J. Gastala, X C. Krumpelman, X E.N. McComb, and X A.W. Korutz ABSTRACT SUMMARY: Functional MR imaging is being performed with increasing frequency in the typical neuroradiology practice; however, many readers of these studies have only a limited knowledge of the functional anatomy of the brain. This text will delineate the locations, anatomic boundaries, and functions of the cortical regions of the brain most commonly encountered in clinical practice—specifically, the regions involved in movement and language. ABBREVIATIONS: FFA ϭ fusiform face area; IPL ϭ inferior parietal lobule; PPC ϭ posterior parietal cortex; SMA ϭ supplementary motor area; VOTC ϭ ventral occipitotemporal cortex his article serves as a review of the functional areas of the brain serving to analyze spatial position and the ventral stream working Tmost commonly mapped during presurgical fMRI studies, to identify what an object is. Influenced by the dorsal and ventral specifically targeting movement and language. We have compiled stream model of vision, Hickok and Poeppel2 hypothesized a sim- what we hope is a useful, easily portable, and concise resource that ilar framework for language. In this model, the ventral stream, or can be accessible to radiologists everywhere. We begin with a re- lexical-semantic system, is involved in sound-to-meaning map- view of the language-processing system. Then we describe the pings associated with language comprehension and semantic ac- gross anatomic boundaries, organization, and function of each cess. -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases.