Increased Functional Connectivity of the Posterior Cingulate Cortex with the Lateral Orbitofrontal Cortex in Depression Wei Cheng1, Edmund T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment
The Journal of Neuroscience, December 1989, 9(12): 4355-4370 Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment Stuart Zola-Morgan,’ Larry Ft. Squire,’ David G. Amaral,2 and Wendy A. Suzuki2J Veterans Administration Medical Center, San Diego, California, 92161, and Department of Psychiatry, University of California, San Diego, La Jolla, California 92093, The Salk Institute, San Diego, California 92136, and 3Group in Neurosciences, University of California, San Diego, La Jolla, California 92093 In monkeys, bilateral damage to the medial temporal region Moss, 1984). (In this notation, H refers to the hippocampus, A produces severe memory impairment. This lesion, which in- to the amygdala, and the plus superscript (+) to the cortical cludes the hippocampal formation, amygdala, and adjacent tissue adjacent to each structure.) This lesion appears to con- cortex, including the parahippocampal gyrus (the H+A+ le- stitute an animal model of medial temporal lobe amnesia like sion), appears to constitute an animal model of human me- that exhibited by the well-studied patient H.M. (Scoville and dial temporal lobe amnesia. Reexamination of histological Milner, 1957). material from previously studied monkeys with H+A+ lesions The H+A+ lesion produces greater memory impairment than indicated that the perirhinal cortex had also sustained sig- a lesion limited to the hippocampal formation and parahip- nificant damage. Furthermore, recent neuroanatomical stud- pocampal cortex-the H+ lesion (Mishkin, 1978; Mahut et al., ies show that the perirhinal cortex and the closely associated 1982; Zola-Morgan and Squire, 1985, 1986; Zola-Morgan et al., parahippocampal cortex provide the major source of cortical 1989a). -

Neural Mechanisms of Navigation Involving Interactions of Cortical and Subcortical Structures
J Neurophysiol 119: 2007–2029, 2018. First published February 14, 2018; doi:10.1152/jn.00498.2017. REVIEW Where Are You Going? The Neurobiology of Navigation Neural mechanisms of navigation involving interactions of cortical and subcortical structures James R. Hinman, Holger Dannenberg, Andrew S. Alexander, and Michael E. Hasselmo Center for Systems Neuroscience, Boston University, Boston, Massachusetts Submitted 5 July 2017; accepted in final form 1 February 2018 Hinman JR, Dannenberg H, Alexander AS, Hasselmo ME. Neural mecha- nisms of navigation involving interactions of cortical and subcortical structures. J Neurophysiol 119: 2007–2029, 2018. First published February 14, 2018; doi: 10.1152/jn.00498.2017.—Animals must perform spatial navigation for a range of different behaviors, including selection of trajectories toward goal locations and foraging for food sources. To serve this function, a number of different brain regions play a role in coding different dimensions of sensory input important for spatial behavior, including the entorhinal cortex, the retrosplenial cortex, the hippocampus, and the medial septum. This article will review data concerning the coding of the spatial aspects of animal behavior, including location of the animal within an environment, the speed of movement, the trajectory of movement, the direction of the head in the environment, and the position of barriers and objects both relative to the animal’s head direction (egocentric) and relative to the layout of the environment (allocentric). The mechanisms for coding these important spatial representations are not yet fully understood but could involve mechanisms including integration of self-motion information or coding of location based on the angle of sensory features in the environment. -

Sustained Activities and Retrieval in a Computational Model of Perirhinal
Sustained activities and retrieval in a computational model of perirhinal cortex Julien Vitay and Fred H. Hamker∗ Abstract Perirhinal cortex is involved in object recognition and novelty detection, but also in multimodal in- tegration, reward association and visual working memory. We propose a computational model that focuses on the role of perirhinal cortex in working memory, particularly with respect to sustained activities and memory retrieval. This model describes how different partial informations are inte- grated into assemblies of neurons that represent the identity of an object. Through dopaminergic modulation, the resulting clusters can retrieve the global information with recurrent interactions between neurons. Dopamine leads to sustained activities after stimulus disappearance that form the basis of the involvement of perirhinal cortex in visual working memory processes. The infor- mation carried by a cluster can also be retrieved by a partial thalamic or prefrontal stimulation. Thus, we suggest that areas involved in planning and memory coordination encode a pointer to access the detailed information encoded in associative cortex such as perirhinal cortex. ∗Allgemeine Psychologie, Psychologisches Institut II, Westf. Wilhelms-Universit¨at M¨unster, Germany 1 Introduction Perirhinal cortex (PRh), composed of cortical areas 35 and 36, is located in the ventromedial part of the temporal lobe. It receives its major inputs from areas TE and TEO of inferotemporal cortex, as well as from entorhinal cortex (ERh), parahippocampal cortex, insular cortex and orbitofrontal cortex (Suzuki & Amaral, 1994). As part of the medial temporal lobe system (with hippocampus and ERh), its primary role is considered to be object-recognition memory, as shown by impairements in delayed matching-to-sample (DMS) or delayed nonmatching-to-sample (DNMS) tasks following PRh cooling or removal (Horel, Pytko-Joiner, Voytko, & Salsbury, 1987; Zola-Morgan, Squire, Amaral, & Suzuki, 1989; Meunier, Bachevalier, Mishkin, & Murray, 1993; Buffalo, Reber, & Squire, 1998). -

Brain Sulci and Gyri: a Practical Anatomical Review
Journal of Clinical Neuroscience 21 (2014) 2219–2225 Contents lists available at ScienceDirect Journal of Clinical Neuroscience journal homepage: www.elsevier.com/locate/jocn Neuroanatomical study Brain sulci and gyri: A practical anatomical review ⇑ Alvaro Campero a,b, , Pablo Ajler c, Juan Emmerich d, Ezequiel Goldschmidt c, Carolina Martins b, Albert Rhoton b a Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina b Department of Neurological Surgery, University of Florida, Gainesville, FL, USA c Department of Neurological Surgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina d Department of Anatomy, Universidad de la Plata, La Plata, Argentina article info abstract Article history: Despite technological advances, such as intraoperative MRI, intraoperative sensory and motor monitor- Received 26 December 2013 ing, and awake brain surgery, brain anatomy and its relationship with cranial landmarks still remains Accepted 23 February 2014 the basis of neurosurgery. Our objective is to describe the utility of anatomical knowledge of brain sulci and gyri in neurosurgery. This study was performed on 10 human adult cadaveric heads fixed in formalin and injected with colored silicone rubber. Additionally, using procedures done by the authors between Keywords: June 2006 and June 2011, we describe anatomical knowledge of brain sulci and gyri used to manage brain Anatomy lesions. Knowledge of the brain sulci and gyri can be used (a) to localize the craniotomy procedure, (b) to Brain recognize eloquent areas of the brain, and (c) to identify any given sulcus for access to deep areas of the Gyri Sulci brain. Despite technological advances, anatomical knowledge of brain sulci and gyri remains essential to Surgery perform brain surgery safely and effectively. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Metabolic Reduction in the Posterior Cingulate Cortex in Very Early Alzheimer’S Disease
Metabolic Reduction in the Posterior Cingulate Cortex in Very Early Alzheimer’s Disease Satoshi Minoshima, MD, PhD,* Bruno Giordani, PhD,t Stanley Berent, PhD,t$ Kirk A. Frey, MD, PhD,*$ Norman L. Foster, MD,$ and David E. Kuhl, MD* This study investigated cerebral glucose metabolism in very early Alzheimer’s disease, before a clinical diagnosis of probable Alzheimer’s disease is possible, using [ ‘8F]flu~r~de~xygluc~~epositron emission tomography. First, 66 patients with probable Alzheimer’s disease with a spectrum of dementia severity (Mini-Mental State Examination score, 0-23) were recruited and studied. Cortical metabolic activity was analyzed topographically using three-dimensional stereotactic surface projections. Regression analysis was performed for each brain pixel to predict metabolic patterns of very early disease. Predictions were tested prospectively in a group of 8 patients who complained only of memory impairment without general cognitive decline (Mini-Mental State Examination score, 25 Ifr 1) at the time of scanning but whose condition later progressed to probable Alzheimer’s disease. Both results were compared to cerebral metabolic activity in 22 age-similar normal control subjects. Prediction and analysis of actual patients consistently indicated marked metabolic reduction (21-22%) in the posterior cingulate cortex and cinguloparietal transitional area in patients with very early Alzheimer’s disease. Mean metabolic reduction in the posterior cingulate cortex was significantly greater than that in the lateral neocortices or parahippocampal cortex. The result suggests a functional importance for the posterior cingulate cortex in impairment of learning and memory, which is a feature of very early Alzheimer’s disease. Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. -

Introduction and Methods the Field of Neuroesthetics Is a Recent Marriage
Introduction and Methods The field of neuroesthetics is a recent marriage of the realms of neuroscience and art. The objective of neuroesthetics is to comprehend the perception and subjective experience of art in terms of their neural substrates. In this study, we examined the effect of a number of original pieces of art on the brain of the artist herself and on that of a novice as she experienced the artwork for the first time. This allowed us to compare neural responses not only amongst different visual conditions but also between an expert (i.e. the artist) and a novice. Lia Cook lent her artwork to be used in this study. The pieces, which she believes to have an innate emotional quality, are cotton and rayon textiles. All of the pieces are portraits with a somewhat abstract, pixelated appearance imparted by the medium. In order to better understand the neural effects of the woven facial images, we used several types of control images. These included (i) scrambled woven pieces, or textiles that were controlled for color, contrast, and size but contained no distinct facial forms; and (ii) photographs, which were all photographs of human faces but were printed on heavy paper and lacked the texture and unique visual appearance of the textiles. All of the pieces are 12.5 in. x 18 in. The expert and novice were each scanned with functional MRI while they viewed and touched the tapestries and photographs. The subjects completed 100 trials divided across two functional scans. Each trial lasted a total of 12 s, with jittered intervals of 4 s to 6 s between trials. -

Reversible Verbal and Visual Memory Deficits After Left Retrosplenial Infarction
Journal of Clinical Neurology / Volume 3 / March, 2007 Case Report Reversible Verbal and Visual Memory Deficits after Left Retrosplenial Infarction Jong Hun Kim, M.D.*, Kwang-Yeol Park, M.D.†, Sang Won Seo, M.D.*, Duk L. Na, M.D.*, Chin-Sang Chung, M.D.*, Kwang Ho Lee, M.D.*, Gyeong-Moon Kim, M.D.* *Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea †Department of Neurology, Chung-Ang University Medical Center, Chung-Ang University School of Medicine, Seoul, Korea The retrosplenial cortex is a cytoarchitecturally distinct brain structure located in the posterior cingulate gyrus and bordering the splenium, precuneus, and calcarine fissure. Functional imaging suggests that the retrosplenium is involved in memory, visuospatial processing, proprioception, and emotion. We report on a patient who developed reversible verbal and visual memory deficits following a stroke. Neuro- psychological testing revealed both anterograde and retrograde memory deficits in verbal and visual modalities. Brain diffusion-weighted and T2-weighted magnetic resonance imaging (MRI) demonstrated an acute infarction of the left retrosplenium. J Clin Neurol 3(1):62-66, 2007 Key Words : Retrosplenium, Memory, Amnesia We report on a patient who developed both verbal INTRODUCTION and visual memory deficits after an acute infarction of the retrosplenial cortex. The main structures related to human memory are the Papez circuit, the basolateral limbic circuit, and the basal forebrain, which communicate with each other through white-matter tracts. Damage to these structures (in- cluding the communication tracts) from hemorrhages, infarctions, and tumors can result in memory dis- turbances.1,2 In addition to these structures, Valenstein et al. -
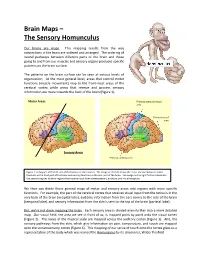
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Differential Cortical Atrophy in Subgroups of Mild Cognitive Impairment
ORIGINAL CONTRIBUTION Differential Cortical Atrophy in Subgroups of Mild Cognitive Impairment Sandra Bell-McGinty, PhD; Oscar L. Lopez, MD; Carolyn Cidis Meltzer, MD; Joelle M. Scanlon, PhD; Ellen M. Whyte, MD; Steven T. DeKosky, MD; James T. Becker, PhD Objective: To compare gray matter brain volumes in jects. Compared with patients with MCI-MCD, patients patients diagnosed with subtypes of mild cognitive im- with MCI-A had significant volume loss of the left ento- pairment (MCI) (those with a focal amnestic disorder and rhinal cortex and inferior parietal lobe. Compared with those with more diffuse cognitive dysfunction) with those patients with MCI-A, patients with MCI-MCD had sig- of elderly controls. nificantly reduced volume of the right inferior frontal gy- rus, right middle temporal gyrus, and bilateral superior Design: Magnetic resonance imaging volumetric study temporal gyrus. Patients with MCI who progressed to Alz- of MCI subgroups (MCI-amnestic [MCI-A], and MCI- heimer disease during follow-up (mean interval 2 years, multiple cognitive domain [MCI-MCD]) using a whole maximum 4.5 years), showed greater atrophy in the left brain voxel-based analysis. entorhinal cortex, bilateral superior temporal gyri, and right inferior frontal gyrus compared with those who did Setting: Referral dementia clinic. not progress. Patients: Thirty-seven patients with MCI (age range, Conclusions: These data provide evidence of distinct 49-85 years; MCI-A, n=9; MCI-MCD, n=28) and 47 con- brain structural abnormalities in 2 groups of patients with trol subjects (age range, 55-81 years). MCI. While both have mesial temporal and cortical vol- ume loss, those with a focal memory deficit have more Main Outcome Measures: Volumetric anatomical mag- involvement of the mesial temporal structures and less netic resonance imaging differences between MCI sub- involvement of the neocortical heteromodal association groups and normal controls, and between patients with areas than those patients with MCI with diffuse cogni- MCI who progressed to dementia.