A Review on Ultrasonic Neuromodulation of the Peripheral Nervous System: Enhanced Or Suppressed Activities?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuropeptide Y1 Receptor Antagonist but Not Neuropeptide Y Itself Increased Bone Mineral Density When Locally Injected with Hyaluronic Acid in Male Wistar Rats
Turkish Journal of Medical Sciences Turk J Med Sci (2020) 50: 1454-1460 http://journals.tubitak.gov.tr/medical/ © TÜBİTAK Research Article doi:10.3906/sag-2001-268 Neuropeptide Y1 receptor antagonist but not neuropeptide Y itself increased bone mineral density when locally injected with hyaluronic acid in male Wistar rats 1, 2 3 Muhammer Özgür ÇEVİK *, Petek KORKUSUZ , Feza KORKUSUZ 1 Department of Medical Genetics, Faculty of Medicine, Adıyaman University, Adıyaman, Turkey 2 Department of Histology and Embryology, Faculty of Medicine, Hacettepe University, Ankara, Turkey 3 Department of Sports Medicine, Faculty of Medicine, Hacettepe University, Ankara, Turkey Received: 31.01.2020 Accepted/Published Online: 19.05.2020 Final Version: 26.08.2020 Background/aim: The nervous system controls bone mass via both the central (CNS) and the peripheral (PNS) nervous systems. Intriguingly, neuropeptide Y (NPY) signaling occurs in both. Less is known on how the PNS stimulated NPY signaling controls bone metabolism. The objective of this study was to evaluate whether NPY or NPY1 receptor antagonist changes local bone mineral density (BMD) when injected into a Wistar rat tibia. Materials and methods: Tibial intramedullary area of 24 wild type male Wistar rats (average weight = 350 ± 50 g, average age = 4 ± 0.5 months) were injected with NPY (1 × 10-5 M and 1 × 10-6 M) and NPY1 receptor antagonist (1 × 10-4 M) dissolved in hyaluronic acid (HA) separately. Tibiae were collected after one and two weeks. BMD was measured with dual-energy X-ray absorptiometry (DXA) and micro quantitative computer tomography (QCT). Histological changes were analyzed with light microscopy, Goldner's Masson trichrome (MT), and hematoxylin-eosin staining. -

Nerve Ultrasound in Dorsal Root Ganglion Disorders: Smaller Nerves Lead to Bigger Insights
Clinical Neurophysiology 130 (2019) 550–551 Contents lists available at ScienceDirect Clinical Neurophysiology journal homepage: www.elsevier.com/locate/clinph Editorial Nerve ultrasound in dorsal root ganglion disorders: Smaller nerves lead to bigger insights See Article, pages 568–572 After decades of having to make do with electric stimulation representing the fascicles, bundled together in a large outer cable and recording (i.e. nerve conduction studies, electromyography sheath (van Alfen et al., 2018). and evoked potentials), nerve ultrasound now provides the oppor- Next, it is important to realize what the ratio between axon/ tunity to improve neurodiagnostic patient care by deploying a myelin and connective tissue in a given nerve segment is, and powerful tool to detect neuromuscular pathology in an accurate how that ratio changes from the proximal root to the distal end and patient-friendly way (Mah et al., 2018; Walker et al., 2018). branches (Schraut et al., 2016). Connective tissue elements of the Nerve ultrasound is also increasingly providing neurologists and perineurium and epineurium are relatively sparse at the very prox- clinical neurophysiologists with the opportunity to increase their imal root and plexus levels, with an average connective tissue con- insight in the pathophysiology of peripheral nervous system tent of around 25–30%. Ultrasonographically, this means that roots (PNS) pathology. In this issue of Clinical Neurophysiology, Leadbet- will always look rather black in appearance without much dis- ter and coworkers (Leadbetter et al., 2019) describe the results of cernible fascicular architecture, as the sparseness of connective tis- their study on nerve ultrasound for diagnosing sensory neuronopa- sue elements provides relatively few reflectors to create an image thy in spinocerebellar ataxia type 2 and CANVAS syndrome. -

Eye Fields in Ocular Decision Making Contrasting the Roles of The
Contrasting the roles of the supplementary and frontal eye fields in ocular decision making Shun-nan Yang and Stephen Heinen J Neurophysiol 111:2644-2655, 2014. First published 26 March 2014; doi:10.1152/jn.00543.2013 You might find this additional info useful... This article cites 42 articles, 19 of which can be accessed free at: /content/111/12/2644.full.html#ref-list-1 Updated information and services including high resolution figures, can be found at: /content/111/12/2644.full.html Additional material and information about Journal of Neurophysiology can be found at: http://www.the-aps.org/publications/jn This information is current as of July 30, 2014. Downloaded from on July 30, 2014 Journal of Neurophysiology publishes original articles on the function of the nervous system. It is published 12 times a year (monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991. Copyright © 2014 by the American Physiological Society. ISSN: 0022-3077, ESSN: 1522-1598. Visit our website at http://www.the-aps.org/. J Neurophysiol 111: 2644–2655, 2014. First published March 26, 2014; doi:10.1152/jn.00543.2013. Contrasting the roles of the supplementary and frontal eye fields in ocular decision making Shun-nan Yang1,2 and Stephen Heinen2 1Vision Performance Institute, College of Optometry, Pacific University, Forest Grove, Oregon; and 2Smith-Kettlewell Eye Research Institute, San Francisco, California Submitted 29 July 2013; accepted in final form 25 March 2014 Yang SN, Heinen S. Contrasting the roles of the supplementary and specified by the motion stimulus (Britten et al. -

Mechanosensitivity and Mechanotransduction Series: Mechanosensitivity in Cells and Tissues, Vol
A. Kamkin, I. Kiseleva (Eds.) Mechanosensitivity and Mechanotransduction Series: Mechanosensitivity in Cells and Tissues, Vol. 4 ▶ One book provides a detailed description of molecular mechanisms of mechanosensing and mechanotransduction in different cells ▶ One book brings together a comprehensive outline of modern vision of structure and functions of cytoskeleton ▶ Provides a wide and detailed description of various signaling pathways This book presents the latest findings in the field of research of mechanosensitivity and mechanotransduction in different cells and tissues. Mechanosensitivity and mechanotransduction of the heart and vascular cells, in the lung, in bone and joint tissues, in sensor systems and in blood cells are described in detail. This Volume focuses on molecular mechanisms 2011, XXIV, 371 p. of mechanosensitivity and mechanotransduction via cytoskeleton. Integrin-mediated mechanotransduction, the role of actin cytoskeleton and the role of other cytoskeletal elements are discussed. It contains a detailed description of several stretch-induced Printed book signaling cascades with multiple levels of crosstalk between different pathways. It Hardcover contains a description of the role of nitric oxide in regulation of cardiac activity and in ISBN 978-90-481-9880-1 regulation of mechanically gated channels in the heart. In the heart mechanical signals are propagated into the intracellular space primarily via integrin-linked complexes, ▶ 219,99 € | £199.99 and are subsequently transmitted from cell to cell via paracrine signaling. Biochemical ▶ *235,39 € (D) | 241,99 € (A) | CHF 259.50 signals derived from mechanical stimuli activate both acute phosphorylation of signaling cascades, such as in the PI3K, FAK, and ILK pathways, and long-term morphological modii cations via intracellular cytoskeletal reorganization and extracellular matrix remodelling. -

Practicerelated Changes in Neural Activation Patterns Investigated Via
r Human Brain Mapping 000:000–000 (2012) r Practice-Related Changes in Neural Activation Patterns Investigated via Wavelet-Based Clustering Analysis Jinae Lee,1 Cheolwoo Park,1 Kara A. Dyckman,2,3,4 Nicole A. Lazar,1 Benjamin P. Austin,5 Qingyang Li,6 and Jennifer E. McDowell2,3,4* 1Department of Statistics, University of Georgia (UGA), Athens, Georgia 2Department of Psychology, University of Georgia (UGA), Athens, Georgia 3Department of Neuroscience, University of Georgia (UGA), Athens, Georgia 4The Bio-Imaging Research Center, University of Georgia (UGA), Athens, Georgia 5UW Cardiovascular Research Center, University of Wisconsin School of Medicine and Public Health and theDepartment of Veterans Affairs (VA) Geriatric Research, Education and Clinical Center (GRECC), Madison, WI, USA 6Center for the Developing Brain, Child Mind Institute, 445 Park Ave, New York, NY 10022, USA r r Abstract: Objectives: To evaluate brain activation using functional magnetic resonance imaging (fMRI) and specifically, activation changes across time associated with practice-related cognitive control during eye movement tasks. Experimental design: Participants were engaged in antisaccade performance (gener- ating a glance away from a cue) while fMR images were acquired during two separate test sessions: (1) at pre-test before any exposure to the task and (2) at post-test, after 1 week of daily practice on antisac- cades, prosaccades (glancing toward a target), or fixation (maintaining gaze on a target). Principal obser- vations: The three practice groups were compared across the two test sessions, and analyses were conducted via the application of a model-free clustering technique based on wavelet analysis. This se- ries of procedures was developed to avoid analysis problems inherent in fMRI data and was composed of several steps: detrending, data aggregation, wavelet transform and thresholding, no trend test, prin- cipal component analysis (PCA), and K-means clustering. -

Supplementary Eye Field Encodes Reward Prediction Error
2950 • The Journal of Neuroscience, February 29, 2012 • 32(9):2950–2963 Behavioral/Systems/Cognitive Supplementary Eye Field Encodes Reward Prediction Error NaYoung So1 and Veit Stuphorn1,2 1Department of Neuroscience, The Johns Hopkins University School of Medicine and Zanvyl Krieger Mind/Brain Institute, and 2Department of Psychological and Brain Sciences, The Johns Hopkins University, Baltimore, Maryland 21218 The outcomes of many decisions are uncertain and therefore need to be evaluated. We studied this evaluation process by recording neuronalactivityinthesupplementaryeyefield(SEF)duringanoculomotorgamblingtask.Whilethemonkeysawaitedtheoutcome,SEF neurons represented attributes of the chosen option, namely, its expected value and the uncertainty of this value signal. After the gamble result was revealed, a number of neurons reflected the actual reward outcome. Other neurons evaluated the outcome by encoding the difference between the reward expectation represented during the delay period and the actual reward amount (i.e., the reward prediction error). Thus, SEF encodes not only reward prediction error but also all the components necessary for its computation: the expected and theactualoutcome.ThissuggeststhatSEFmightactivelyevaluatevalue-baseddecisionsintheoculomotordomain,independentofother brain regions. Introduction ent evaluative stages can indicate whether a brain area contains all In most real-life decisions, the outcomes are uncertain or can the neuronal signals sufficient to compute RPE signals. change over time. The values -

Modulation of Interhemispheric Inhibition Between Primary Motor Cortices Induced by Manual Motor Imitation: a Transcranial Magnetic Stimulation Study
brain sciences Article Modulation of Interhemispheric Inhibition between Primary Motor Cortices Induced by Manual Motor Imitation: A Transcranial Magnetic Stimulation Study Dongting Tian 1,*, Shin-ichi Izumi 1,2 and Eizaburo Suzuki 1,3 1 Department of Physical Medicine and Rehabilitation, Tohoku University Graduate School of Medicine, Sendai 980-8575, Japan; [email protected] (S.-i.I.); [email protected] (E.S.) 2 Department of Physical Medicine and Rehabilitation, Tohoku University Graduate School of Biomedical Engineering, Sendai 980-8575, Japan 3 Department of Physical Therapy, Yamagata Prefectural University of Health Sciences, 260 Kamiyanagi, Yamagata 990-2212, Japan * Correspondence: [email protected] Abstract: Imitation has been proven effective in motor development and neurorehabilitation. How- ever, the relationship between imitation and interhemispheric inhibition (IHI) remains unclear. Transcranial magnetic stimulation (TMS) can be used to investigate IHI. In this study, the modifica- tion effects of IHI resulting from mirror neuron system (MNS) activation during different imitations are addressed. We measured IHI between homologous primary motor cortex (M1) by analyzing the ipsilateral silent period (iSP) evoked by single-pulse focal TMS during imitation and analyzed the respective IHI modulation during and after different patterns of imitation. Our main results showed that throughout anatomical imitation, significant time-course changes of iSP duration through the Citation: Tian, D.; Izumi, S.-i.; experiment were observed in both directions. iSP duration declined from the pre-imitation time Suzuki, E. Modulation of Interhemispheric Inhibition between point to the post-imitation time point and did not return to baseline after 30 min rest. -

Functional Role of the Supplementary and Pre-Supplementary Motor Areas
REVIEWS Functional role of the supplementary and pre-supplementary motor areas Parashkev Nachev*‡, Christopher Kennard‡ and Masud Husain* Abstract | The supplementary motor complex consists of the supplementary motor area, the supplementary eye field and the pre-supplementary motor area. In recent years, these areas have come under increasing scrutiny from cognitive neuroscientists, motor physiologists and clinicians because they seem to be crucial for linking cognition to action. However, theories regarding their function vary widely. This Review brings together the data regarding the supplementary motor regions, highlighting outstanding issues and providing new perspectives for understanding their functions. Ever since they were first identified, the regions that which we hope will stimulate the development of comprise the supplementary motor complex (SMC) have conceptual frameworks for a better understanding remained, in large part, a mystery. Most investigators now of this region. appreciate that these brain regions are far from ‘supple- mentary’ to requirements1–7. Without them, there are Anatomy and connections profound alterations in behaviour. For example, lesions to The supplementary motor area (SMA) and pre- these areas in humans can lead to alien-limb syndrome, supplementary motor area (pre-SMA) are, in humans, with patients demonstrating involuntary actions such as located on the medial aspect of the brain: in the dorso- grasping nearby objects — even other people — without medial frontal cortex3,14, anterior to the leg representa- ever intending to do so8,9. Some individuals demonstrate tion of the primary motor cortex (FIG. 1). Both areas lie utilization behaviour: unable to resist the impulse to use in the superior frontal gyrus and constitute the medial an object that has been placed within their reach, even part of Brodmann’s area 6c (later divided into two areas: when the object is not needed10. -
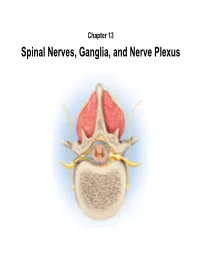
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

The Peripheral Nervous System
The Peripheral Nervous System Dr. Ali Ebneshahidi Peripheral Nervous System (PNS) – Consists of 12 pairs of cranial nerves and 31 pairs of spinal nerves. – Serves as a critical link between the body and the central nervous system. – peripheral nerves contain an outermost layer of fibrous connective tissue called epineurium which surrounds a thinner layer of fibrous connective tissue called perineurium (surrounds the bundles of nerve or fascicles). Individual nerve fibers within the nerve are surrounded by loose connective tissue called endoneurium. Cranial Nerves Cranial nerves are direct extensions of the brain. Only Nerve I (olfactory) originates from the cerebrum, the remaining 11 pairs originate from the brain stem. Nerve I (Olfactory)- for the sense of smell (sensory). Nerve II (Optic)- for the sense of vision (sensory). Nerve III (Oculomotor)- for controlling muscles and accessory structures of the eyes ( primarily motor). Nerve IV (Trochlear)- for controlling muscles of the eyes (primarily motor). Nerve V (Trigeminal)- for controlling muscles of the eyes, upper and lower jaws and tear glands (mixed). Nerve VI (Abducens)- for controlling muscles that move the eye (primarily motor). Nerve VII (Facial) – for the sense of taste and controlling facial muscles, tear glands and salivary glands (mixed). Nerve VIII (Vestibulocochlear)- for the senses of hearing and equilibrium (sensory). Nerve IX (Glossopharyngeal)- for controlling muscles in the pharynx and to control salivary glands (mixed). Nerve X (Vagus)- for controlling muscles used in speech, swallowing, and the digestive tract, and controls cardiac and smooth muscles (mixed). Nerve XI (Accessory)- for controlling muscles of soft palate, pharynx and larynx (primarily motor). Nerve XII (Hypoglossal) for controlling muscles that move the tongue ( primarily motor). -

Systematic Examination of Low-Intensity Ultrasound Parameters
TOOLS AND RESOURCES Systematic examination of low-intensity ultrasound parameters on human motor cortex excitability and behavior Anton Fomenko1†*, Kai-Hsiang Stanley Chen1,2†, Jean-Franc¸ois Nankoo1, James Saravanamuttu1, Yanqiu Wang1, Mazen El-Baba1, Xue Xia3, Shakthi Sanjana Seerala4, Kullervo Hynynen4, Andres M Lozano1,5*, Robert Chen1,3* 1Krembil Research Institute, University Health Network, Toronto, Canada; 2Department of Neurology, National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan; 3Division of Neurology, Department of Medicine, University of Toronto, Toronto, Canada; 4Sunnybrook Research Institute, Toronto, Canada; 5Division of Neurosurgery, Department of Surgery, Toronto Western Hospital, University of Toronto, Toronto, Canada Abstract Low-intensity transcranial ultrasound (TUS) can non-invasively modulate human neural activity. We investigated how different fundamental sonication parameters influence the effects of TUS on the motor cortex (M1) of 16 healthy subjects by probing cortico-cortical excitability and behavior. A low-intensity 500 kHz TUS transducer was coupled to a transcranial magnetic stimulation (TMS) coil. TMS was delivered 10 ms before the end of TUS to the left M1 hotspot of the first dorsal interosseous muscle. Varying acoustic parameters (pulse repetition frequency, duty *For correspondence: cycle, and sonication duration) on motor-evoked potential amplitude were examined. Paired-pulse [email protected] measures of cortical inhibition and facilitation, and performance on a visuomotor task was also (AF); assessed. TUS safely suppressed TMS-elicited motor cortical activity, with longer sonication [email protected] (AML); durations and shorter duty cycles when delivered in a blocked paradigm. TUS increased GABA - [email protected] (RC) A mediated short-interval intracortical inhibition and decreased reaction time on visuomotor task but † These authors contributed not when controlled with TUS at near-somatosensory threshold intensity. -

Short- and Long-Term Deficits After Unilateral Ablation Andthe Effects of Subsequent Callosal Section’
0270.6474/84/0404-0918$02.00/0 The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 4, pp. 918-929 Printed in U.S.A. April 1984 SUPPLEMENTARY MOTOR AREA OF THE MONKEY’S CEREBRAL CORTEX: SHORT- AND LONG-TERM DEFICITS AFTER UNILATERAL ABLATION ANDTHE EFFECTS OF SUBSEQUENT CALLOSAL SECTION’ COBIE BRINKMAN Experimental Neurology Unit, The John Curtin School of Medical Research, Australian National University, Canberra, Australia 2601 Received March 7, 1983; Revised November 11, 1983; Accepted November 15, 1983 Abstract The short-term and long-term behavioral effects of unilateral lesions of the supplementary motor area (SMA) were studied in five monkeys (Mczcaca fascicularis ssp). A monkey with a unilateral lesion of the premotor area (PM) served as a control. In all animals, general behavior was unaffected by the lesions. For a few weeks postoperatively, all monkeys showed a clumsiness of forelimb movements, bilaterally, which involved both the distal and proximal muscles. Two SMA-lesioned monkeys (but not the PM-lesioned one), studied for up to 1 year postoperatively, showed a characteristic deficit of bimanual coordination where the two hands tended to behave in a similar manner instead of sharing the task between them. This deficit was more pronounced after a lesion contralateral to the nonpreferred hand. The deficit was interpreted as indicating that the intact SMA now influenced the motor outflow of both the ipsilateral hemisphere and the contralateral one through the corpus callosum. Callosal section immediately abolished the bimanual deficit, although the clumsiness returned transiently. The results imply that SMA may give rise normally to discharges informing the contralateral hemisphere of intended and/or ongoing movements via the corpus callosum.