Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -
Core-Example1.Pdf
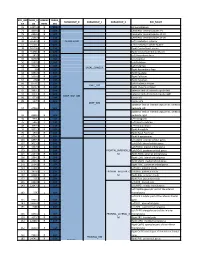
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Supplemental Data
Supplemental Figure 1. Representative clinical 18F-FDG brain PET images from two extremes of image evaluation. The images shown are (A) low-dose, (B) deep learning prediction in image space, (C) deep learning prediction in sinogram space, (D) full-dose. The red numbers indicate scores assigned by one expert physician to images. THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. STD of SUV bias SUV bias(%) White matter Third ventricle Temporal horn Frontal Horn Brainstem Cerebellum Cingulate gyrus, pos Cingulate gyrus, ant Insula Substantia nigra Corp Callosum Pallidum Thalamus Putamen Nucleus accumbens Caudate nucleus Cuneus Lingual gyrus Lat occipital lobe Inf parietal lobe Superior parietal gyrus Anterior Sup Temporal Gyrus Posterior temporal lobe Fusiform Mid & Inf temporal gyrus Superior temporal gyrus Para hippocampal Temporal lobe, lateral Temporal lobe, medial Amygdala Hippocampus Subgenual frontal Subcallosal area Subgenual frontal cortex Posterior orbital gyrus Lateral orbital gyrus Medial orbital gyrus Superior frontal gyrus Inferior frontal gyrus Anterior orbital gyrus Straight gyrus Precentral gyrus Middlle frontal gyrus 0 0.5 1 1.5 2 -3 -1 1 3 LD PIS PSS LD PIS PSS THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. Supplemental Figure 2. Plots of SUV bias standard deviation (left panel) and mean SUV bias (%) (right panel) in the different brain regions for LD, PIS, and PSS PET images. The left and right regions were merged, thus reducing the number of brain regions to 44. THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. THE JOURNAL OF NUCLEAR MEDICINE • Vol. -

Anatomic Moment Limbic System Anatomy: an Overview
Anatomic Moment Limbic System Anatomy: An Overview Leighton P. Mark,1.3 David L. Daniels, 1 Thomas P. Naidich,2 and Jessica A. Borne1 The anatomy of the limbic system has become shaped sulcus (Fig. 2) designated as 1) the hip more relevant due to the development of high pocampal fissure where it lies superior to the resolution and functional magnetic resonance parahippocampal gyrus, and 2) the callosal sulcus (MR) imaging. This review is the first of a series where it lies inferior to the cingulate gyrus of Anatomic Moments designed to highlight se (Fig. 3). lected features of limbic system anatomy in order Nested within this )-shaped sulcus is another ) to facilitate their application to MR interpretation. shaped structure formed by 1) the dentate gyrus Limbic system terminology of early anatomists and hippocampus in the temporal lobe (Figs. 2 was largely descriptive (Table 1), but the nomen and 4), 2) the hippocampal tail which consists of clature used in this series will follow that of recent thin gray and white matter structures located just authors (Table 2) (1-7). posterior and inferior to the splenium of the In accordance with the curvilinear development corpus callosum (Fig. 5), and 3) the supracallosal of the cerebral hemispheres (Fig. 1), the struc gyrus which is located inferior to the callosal tures of the limbic lobe form a series of "nested" sulcus. The supracallosal gyrus is intimately ap )-shaped curves (Fig. 2). plied to the upper surface of the corpus callosum, The widest curve is formed by the large limbic and contains gray matter termed the indusium gyrus which is designated as 1) the parahippo griseum (Fig. -

031909.M1-Cns.Limbicsystem.Pdf
Attribution: Department of Neurology, 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution–Non-commercial–Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/ We have reviewed this material in accordance with U.S. Copyright Law and have tried to maximize your ability to use, share, and adapt it. The citation key on the following slide provides information about how you may share and adapt this material. Copyright holders of content included in this material should contact [email protected] with any questions, corrections, or clarification regarding the use of content. For more information about how to cite these materials visit http://open.umich.edu/education/about/terms-of-use. Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have questions about your medical condition. Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers. Citation Key for more information see: http://open.umich.edu/wiki/CitationPolicy Use + Share + Adapt { Content the copyright holder, author, or law permits you to use, share and adapt. } Public Domain – Government: Works that are produced by the U.S. Government. (USC 17 § 105) Public Domain – Expired: Works that are no longer protected due to an expired copyright term. Public Domain – Self Dedicated: Works that a copyright holder has dedicated to the public domain. -

Cortex Monkey
NEURONOGRAPHIC ANALYSIS OF MEDIAL AND BASAL CEREBRAL CORTEX. II. MONKEY KARL H. PRIBRAM AND PAUL D. MACLEAN Reprinted from J. Neurophysiol., 1953,16: 324-340 .' NEURONOGRAPHIC ANALYSIS OF MEDIAL AND BASAL CEREBRAL CORTEX. II. MONKEYl KARL H. PRIBRAM2 AND PAUL D. MAcLEAN Laboratory of Physiology and Department of Psychiatry, Yale University, New Haven, Connecticut ATTEMPTS TO ANALYZE the highly complex behavioral effects of large cere brallesions of the frontal and temporal lobes have emphasized the need for more information regarding the functional organization of the cortex on the medial and basal aspects of the hemispheres. Comparative, ontogenetic, and cytoarchitectural studies have drawn a distinction between the cortex sur rounding the hilus of each hemisphere, known as the great limbic lobe (6), and the rest of'the cerebral mantle. It has consequently been postulated that lesions limited to the limbic system and the adjacent related cortex would result in behavioral changes distinctive from those restricted to the phylo genetically newer part of the forebrain. In order to test this hypothesis and to have a guide for making lesions, knowledge was required concerning the related areas of limbic and extralimbic cortex. The method of physiological neuronography was applied in this study for obtaining such information. In both cat and monkey five corresponding large regions, each including limbic and extralimbic cortex, were delineated. The experiments on cat were reported in the preceding communication. This paper deals with the findings in monkey. MATERIALS, METHODS, AND PROCEDURES Twenty Rhesus macacus monkeys were used. Anesthesia was produced with Dial with urethane,3 half given intravenously and half intraperitoneally, in a dosage of 0.4 cc./kg. -

Primate Anterior Cingulate Cortex: Where Motor Control Drive And
REVIEWS PRIMATE ANTERIOR CINGULATE CORTEX: WHERE MOTOR CONTROL, DRIVE AND COGNITION INTERFACE Tomá˘sPaus Controversy surrounds the function of the anterior cingulate cortex. Recent discussions about its role in behavioural control have centred on three main issues: its involvement in motor control, its proposed role in cognition and its relationship with the arousal/drive state of the organism. I argue that the overlap of these three domains is key to distinguishing the anterior cingulate cortex from other frontal regions, placing it in a unique position to translate intentions to actions. 1 TEMPERAMENT AND The past decade saw a surge of interest in the structure sulcus, which is present in only 30–50% of individuals . CHARACTER INVENTORY and function of the anterior cingulate cortex (ACC) in The incidence1–3 and volume4 of the paracingulate sul- A self-report measure of seven human and non-human primates. Three key features cus are greater in the left hemisphere; this asymmetry basic dimensions of dominate current discussions on the role of the ACC in might be related to the involvement of the paracingu- temperament (novelty seeking, 5 harm avoidance, reward behavioural control. First, dense projections from the late region in speech . Conversely, the volume of grey dependence and persistence) ACC to the motor cortex and spinal cord seem to impli- matter buried in the anterior section of the cingulate and character (self-directedness, cate this region in motor control. Second, reciprocal cor- sulcus is greater in the right hemisphere, as compared cooperativeness and self- tico–cortical connections of the ACC with the lateral with the left4. -

01 06 Medial Surface of the Brain-NOTES.Pdf
Medial Surface of the Brain Medical Neuroscience | Tutorial Notes Medial Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the medial view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. In this set of tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Microsurgical Anatomy of the Subcallosal Artery
Surgical and Radiologic Anatomy (2019) 41:1037–1044 https://doi.org/10.1007/s00276-019-02279-8 ANATOMIC VARIATIONS Microsurgical anatomy of the subcallosal artery Louis Chenin1,2 · Aimé Kaoudi1,2 · Pascal Foulon1 · Eric Havet1 · Johann Peltier1,2 Received: 3 February 2019 / Accepted: 24 June 2019 / Published online: 27 June 2019 © Springer-Verlag France SAS, part of Springer Nature 2019 Abstract Background and purpose The subcallosal artery [SCA, a branch of the anterior communicating artery (ACoA)] is not well described in the literature. However, the memory disorders that can occur after surgical repair of ruptured ACoA aneurysms might be related to infarction of the SCA. The objective of the present study was to perform a thorough anatomical assess- ment of the SCA. Methods The study was carried out over a 6-month period in a University Hospital’s anatomy laboratory, using brains extracted from human cadavers. The brains were injected with colored neoprene latex and dissected to study the SCA’s origin, path, termination, diameter, length, and vascularized territories. Results 21 cadaveric specimens were studied. The mean ± standard deviation diameter and length of the SCA were 0.83 ± 0.57 mm and 38.14 ± 25.11 mm, respectively. The predominantly vascularized territories were the paraterminal gyrus (100%), the parolfactory gyrus (78.95%), the rostrum (84.21%) and genu (78.95%) of the corpus callosum, the lamina terminalis (78.95%), the anterior commissure (63.16%), the anterior cingulate gyrus (47.37%), and the fornix (26.32%). When the SCA supplied the fornix and the anterior cingulate gyrus, it was signifcantly longer and broader (p < 0.05). -

The Cerebral Sulci and Gyri
Neurosurg Focus 28 (2):E2, 2010 The cerebral sulci and gyri GUILHERME CARVALHAL RIBAS, M.D. Department of Surgery, University of São Paulo Medical School—LIM-02, Hospital Israelita Albert Einstein, São Paulo, Brazil The aim of this study was to describe in detail the microanatomy of the cerebral sulci and gyri, clarifying the nomenclature for microneurosurgical purposes. An extensive review of the literature regarding the historical, evo- lutionary, embryological, and anatomical aspects pertinent to human cerebral sulci and gyri was conducted, with a special focus on microneuroanatomy issues in the field of neurosurgery. An intimate knowledge of the cerebral sulci and gyri is needed to understand neuroimaging studies, as well as to plan and execute current microneurosurgical procedures. (DOI: 10.3171/2009.11.FOCUS09245) KEY WORDS • brain gyrus • brain mapping • brain sulcus • cerebral cortex • cerebral lobe LTHOUGH there is no strict relationship between atized surgical procedure.23 As early as ~ 10,000 years brain structure and function, current knowledge ago, cranial trephination was performed “successfully” shows that the two are closely interrelated. The (that is, with new bone formation after the procedure) in Abrain is divided into regions and subdivided into more the neolithic cultures of Europe, and there are findings specific zones, although there is increasing evidence that dating to 2000 years ago in South America, where the the borders between those zones are much blurrier than practice was particularly common in the pre-Incan