Microsurgical Anatomy of the Subcallosal Artery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Fenestration of the Lamina Terminalis
DORIS DUKE MEDICAL STUDENTS’ JOURNAL Volume IV, 2004-2005 A Prospective, Randomized, Single-Surgeon Trial of Fenestration of the Lamina Terminalis Evan R. Ransom A. Abstract Aneurysmal subarachnoid hemorrhage (aSAH) following ruptured intracranial aneurysm affects approximately 25,000 to 30, 000 people each year. Despite advances in early diagnosis and management, aSAH remains a frequent cause of death and disability. A common complication of this disease is hydrocephalus, a condition where the fluid surrounding the brain does not drain properly, causing an increase in pressure. In order to prevent damage to the brain from hydrocephalus it is often necessary to place a device (shunt) that drains cerebrospinal fluid from around the brain into the abdomen where it can be absorbed. Recent scientific investigations have shown that a procedure performed at the time of surgery for aSAH can decrease the likelihood of developing hydrocephalus requiring a shunt. This procedure involves making a very small connection between one of the fluid spaces in the brain (ventricle) and the fluid surrounding the brain (subarachnoid space). Though the procedure decreases the incidence of shunt-dependent hydrocephalus, its neuropsychological sequellae remain poorly defined. Reducing the incidence of hydrocephalus requiring a shunt is likely to improve patients' quality of life. However, it remains possible that this procedure, which is now widely used, may have unknown adverse effects on emotional or cognitive function. This is a particularly relevant concern given the proximity of the lamina terminalis to important functional regions of the brainstem, forebrain, thalamus, and hypothalamus. We propose to assign patients requiring surgery for aSAH by chance (like a coin-toss) to either: 1) receive this procedure (lamina terminalis fenestration) as part of their surgery; or, 2) undergo surgery without lamina terminalis fenestration. -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P
Cerebral Cortex, November 2015;25: 4351–4373 doi: 10.1093/cercor/bhv019 Advance Access Publication Date: 24 February 2015 Original Article ORIGINAL ARTICLE Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex John P. Aggleton1, Nicholas F. Wright1, Douglas L. Rosene2, and Richard C. Saunders3 1School of Psychology, Cardiff University, Cardiff, Wales CF10 3AT, UK, 2School of Medicine, Department of Anatomy and Neurobiology, Boston University, Boston MA 02118, USA, and 3Laboratory of Neuropsychology, National Institute of Mental Health, Bethesda, MD 20892, USA Address correspondence to: John P. Aggleton, School of Psychology, Cardiff University, Park Place, Cardiff, Wales CF10 3AT, UK. Email: [email protected] Abstract The projections from the amygdala and hippocampus (including subiculum and presubiculum) to prefrontal cortex were compared using anterograde tracers injected into macaque monkeys (Macaca fascicularis, Macaca mulatta). Almost all prefrontal areas were found to receive some amygdala inputs. These connections, which predominantly arose from the intermediate and magnocellular basal nucleus, were particularly dense in parts of the medial and orbital prefrontal cortex. Contralateral inputs were not, however, observed. The hippocampal projections to prefrontal areas were far more restricted, being confined to the ipsilateral medial and orbital prefrontal cortex (within areas 11, 13, 14, 24a, 32, and 25). These hippocampal projections principally arose from the subiculum, with the fornix providing the sole route. Thus, while the lateral prefrontal cortex essentially receives only amygdala inputs, the orbital prefrontal cortex receives both amygdala and hippocampal inputs, though these typically target different areas. Only in medial prefrontal cortex do direct inputs from both structures terminate in common sites. -

The Three Amnesias
The Three Amnesias Russell M. Bauer, Ph.D. Department of Clinical and Health Psychology College of Public Health and Health Professions Evelyn F. and William L. McKnight Brain Institute University of Florida PO Box 100165 HSC Gainesville, FL 32610-0165 USA Bauer, R.M. (in press). The Three Amnesias. In J. Morgan and J.E. Ricker (Eds.), Textbook of Clinical Neuropsychology. Philadelphia: Taylor & Francis/Psychology Press. The Three Amnesias - 2 During the past five decades, our understanding of memory and its disorders has increased dramatically. In 1950, very little was known about the localization of brain lesions causing amnesia. Despite a few clues in earlier literature, it came as a complete surprise in the early 1950’s that bilateral medial temporal resection caused amnesia. The importance of the thalamus in memory was hardly suspected until the 1970’s and the basal forebrain was an area virtually unknown to clinicians before the 1980’s. An animal model of the amnesic syndrome was not developed until the 1970’s. The famous case of Henry M. (H.M.), published by Scoville and Milner (1957), marked the beginning of what has been called the “golden age of memory”. Since that time, experimental analyses of amnesic patients, coupled with meticulous clinical description, pathological analysis, and, more recently, structural and functional imaging, has led to a clearer understanding of the nature and characteristics of the human amnesic syndrome. The amnesic syndrome does not affect all kinds of memory, and, conversely, memory disordered patients without full-blown amnesia (e.g., patients with frontal lesions) may have impairment in those cognitive processes that normally support remembering. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -

Gbx-2, and Writ-3 in the Embryonic Day 12.5 Mouse Forebrain Defines Potential Transverse and Longitudinal Segmental Boundaries
The Journal of Neuroscience, July 1993, 13(7): 31553172 Spatially Restricted Expression of D/x- 1, D/x-Z (Tes- I), Gbx-2, and Writ-3 in the Embryonic Day 12.5 Mouse Forebrain Defines Potential Transverse and Longitudinal Segmental Boundaries Alessandro Bulfone,1~2~4~5Luis Puelles,6 Matthew H. Porteus, 1*2*4*5 Michael A. Frohman,’ Gail R. Martin,3*5 and John L. R. Rubensteini,2*4.5 ‘Neurogenetics Laboratory, Departments of *Psychiatry and 3Anatomy, and Programs in 4Neuroscience and 5Developmental Biology, University of California, San Francisco, California 94143-0984, 6Department of Anatomy, Faculty of Medicine, University of Murcia, Murcia, Spain 30100, and ‘Department of Pharmacology, SUNY, Stonybrook, New York 11794-8651 The expression patterns of four genes that are potential cells differentiate and give rise to different neural structures. For regulators of development were examined in the CNS of the instance, along the A-P axis the neural tube subdivides to form embryonic day 12.5 mouse embryo. Three of the genes, Dlx- the spinal cord, the myelencephalon, the metencephalon, the 1, D/x-Z (Tes- I), and Gbx-2, encode homeodomain-contain- mesencephalon, the diencephalon, and the telencephalon. With- ing proteins, and one gene, Wnf-3, encodes a putative se- in each of these regions, subsequent differentiation of different creted differentiation factor. These genes are expressed in neuroepithelial domains results in neural structures of distinct spatially restricted transverse and longitudinal domains in histologies. For example, as viewed from a cross section of the the embryonic neural tube, and are also differentially ex- embryonic telencephalon, the neocortex derives from the dor- pressed within the wall of the neural tube. -

The Evolutionary Development of the Brain As It Pertains to Neurosurgery
Open Access Original Article DOI: 10.7759/cureus.6748 The Evolutionary Development of the Brain As It Pertains to Neurosurgery Jaafar Basma 1 , Natalie Guley 2 , L. Madison Michael II 3 , Kenan Arnautovic 3 , Frederick Boop 3 , Jeff Sorenson 3 1. Neurological Surgery, University of Tennessee Health Science Center, Memphis, USA 2. Neurological Surgery, University of Arkansas for Medical Sciences, Little Rock, USA 3. Neurological Surgery, Semmes-Murphey Clinic, Memphis, USA Corresponding author: Jaafar Basma, [email protected] Abstract Background Neuroanatomists have long been fascinated by the complex topographic organization of the cerebrum. We examined historical and modern phylogenetic theories pertaining to microneurosurgical anatomy and intrinsic brain tumor development. Methods Literature and history related to the study of anatomy, evolution, and tumor predilection of the limbic and paralimbic regions were reviewed. We used vertebrate histological cross-sections, photographs from Albert Rhoton Jr.’s dissections, and original drawings to demonstrate the utility of evolutionary temporal causality in understanding anatomy. Results Phylogenetic neuroanatomy progressed from the substantial works of Alcmaeon, Herophilus, Galen, Vesalius, von Baer, Darwin, Felsenstein, Klingler, MacLean, and many others. We identified two major modern evolutionary theories: “triune brain” and topological phylogenetics. While the concept of “triune brain” is speculative and highly debated, it remains the most popular in the current neurosurgical literature. Phylogenetics inspired by mathematical topology utilizes computational, statistical, and embryological data to analyze the temporal transformations leading to three-dimensional topographic anatomy. These transformations have shaped well-defined surgical planes, which can be exploited by the neurosurgeon to access deep cerebral targets. The microsurgical anatomy of the cerebrum and the limbic system is redescribed by incorporating the dimension of temporal causality. -
Core-Example1.Pdf
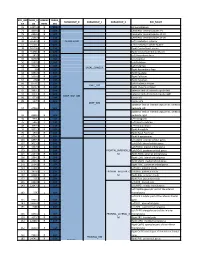
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Supplemental Data
Supplemental Figure 1. Representative clinical 18F-FDG brain PET images from two extremes of image evaluation. The images shown are (A) low-dose, (B) deep learning prediction in image space, (C) deep learning prediction in sinogram space, (D) full-dose. The red numbers indicate scores assigned by one expert physician to images. THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. STD of SUV bias SUV bias(%) White matter Third ventricle Temporal horn Frontal Horn Brainstem Cerebellum Cingulate gyrus, pos Cingulate gyrus, ant Insula Substantia nigra Corp Callosum Pallidum Thalamus Putamen Nucleus accumbens Caudate nucleus Cuneus Lingual gyrus Lat occipital lobe Inf parietal lobe Superior parietal gyrus Anterior Sup Temporal Gyrus Posterior temporal lobe Fusiform Mid & Inf temporal gyrus Superior temporal gyrus Para hippocampal Temporal lobe, lateral Temporal lobe, medial Amygdala Hippocampus Subgenual frontal Subcallosal area Subgenual frontal cortex Posterior orbital gyrus Lateral orbital gyrus Medial orbital gyrus Superior frontal gyrus Inferior frontal gyrus Anterior orbital gyrus Straight gyrus Precentral gyrus Middlle frontal gyrus 0 0.5 1 1.5 2 -3 -1 1 3 LD PIS PSS LD PIS PSS THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. Supplemental Figure 2. Plots of SUV bias standard deviation (left panel) and mean SUV bias (%) (right panel) in the different brain regions for LD, PIS, and PSS PET images. The left and right regions were merged, thus reducing the number of brain regions to 44. THE JOURNAL OF NUCLEAR MEDICINE • Vol. 61• No. 9 • September 2020 Sanaat et al. THE JOURNAL OF NUCLEAR MEDICINE • Vol. -

Anatomic Moment Limbic System Anatomy: an Overview
Anatomic Moment Limbic System Anatomy: An Overview Leighton P. Mark,1.3 David L. Daniels, 1 Thomas P. Naidich,2 and Jessica A. Borne1 The anatomy of the limbic system has become shaped sulcus (Fig. 2) designated as 1) the hip more relevant due to the development of high pocampal fissure where it lies superior to the resolution and functional magnetic resonance parahippocampal gyrus, and 2) the callosal sulcus (MR) imaging. This review is the first of a series where it lies inferior to the cingulate gyrus of Anatomic Moments designed to highlight se (Fig. 3). lected features of limbic system anatomy in order Nested within this )-shaped sulcus is another ) to facilitate their application to MR interpretation. shaped structure formed by 1) the dentate gyrus Limbic system terminology of early anatomists and hippocampus in the temporal lobe (Figs. 2 was largely descriptive (Table 1), but the nomen and 4), 2) the hippocampal tail which consists of clature used in this series will follow that of recent thin gray and white matter structures located just authors (Table 2) (1-7). posterior and inferior to the splenium of the In accordance with the curvilinear development corpus callosum (Fig. 5), and 3) the supracallosal of the cerebral hemispheres (Fig. 1), the struc gyrus which is located inferior to the callosal tures of the limbic lobe form a series of "nested" sulcus. The supracallosal gyrus is intimately ap )-shaped curves (Fig. 2). plied to the upper surface of the corpus callosum, The widest curve is formed by the large limbic and contains gray matter termed the indusium gyrus which is designated as 1) the parahippo griseum (Fig.