OVERVIEW the Cerebral Cortex Is Divided Into 49 Macro-Anatomically
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10041.Full.Pdf
The Journal of Neuroscience, July 23, 2014 • 34(30):10041–10054 • 10041 Systems/Circuits Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders Sarah R. Heilbronner and Suzanne N. Haber Department of Pharmacology and Physiology, University of Rochester Medical Center, Rochester, New York 14642 The cingulum bundle (CB) is one of the brain’s major white matter pathways, linking regions associated with executive function, decision-making, and emotion. Neuroimaging has revealed that abnormalities in particular locations within the CB are associated with specific psychiatric disorders, including depression and bipolar disorder. However, the fibers using each portion of the CB remain unknown. In this study, we used anatomical tract-tracing in nonhuman primates (Macaca nemestrina, Macaca fascicularis, Macaca mulatta)toexaminetheorganizationofspecificcingulate,noncingulatefrontal,andsubcorticalpathwaysthroughtheCB.Thegoalswere as follows: (1) to determine connections that use the CB, (2) to establish through which parts of the CB these fibers travel, and (3) to relate the CB fiber pathways to the portions of the CB identified in humans as neurosurgical targets for amelioration of psychiatric disorders. Results indicate that cingulate, noncingulate frontal, and subcortical fibers all travel through the CB to reach both cingulate and noncin- gulate targets. However, many brain regions send projections through only part, not all, of the CB. For example, amygdala fibers are not present in the caudal portion of the dorsal CB. These results allow segmentation of the CB into four unique zones. We identify the specific connections that are abnormal in psychiatric disorders and affected by neurosurgical interventions, such as deep brain stimulation and cingulotomy. -
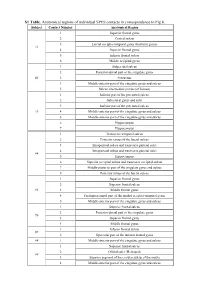
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Acetylcholinesterase Staining in Human Auditory and Language
Acetylcholinesterase Staining in Human Jeffrey J. Hutsler and Michael S. Gazzaniga Auditory and Language Cortices: Center for Neuroscience, University of California, Davis Regional Variation of Structural Features California 95616 Cholinergic innovation of the cerebral neocortex arises from the basal 1974; Greenfield, 1984, 1991; Robertson, 1987; Taylor et al., forebrain and projects to all cortical regions. Acetylcholinesterase 1987; Krisst, 1989; Small, 1989, 1990). AChE-containing axons (AChE), the enzyme responsible for deactivating acetylcholine, is found of the cerebral cortex are also immunoreactive for choline within both cholinergic axons arising from the basal forebrain and a acetyltransferase (ChAT) and are therefore known to be cho- Downloaded from subgroup of pyramidal cells in layers III and V of the cerebral cortex. linergic (Mesulam and Geula, 1992). This pattern of staining varies with cortical location and may contrib- AChE-containing pyramidal cells of layers III and V are not ute uniquely to cortical microcircuhry within functionally distinct cholinergic (Mesulam and Geula, 1991), but it has been sug- regions. To explore this issue further, we examined the pattern of AChE gested that they are cholinoceptive (Krnjevic and Silver, 1965; staining within auditory, auditory association, and putative language Levey et al., 1984; Mesulam et al., 1984b). In support of this regions of whole, postmortem human brains. notion, layer in and V pyramidal cell excitability can be mod- http://cercor.oxfordjournals.org/ The density and distribution of acetylcholine-containing axons and ulated by the application of acetylcholine in the slice prepa- pyramidal cells vary systematically as a function of auditory process- ration (McCormick and Williamson, 1989). Additionally, mus- ing level. -

Gliomas of the Cingulate Gyrus: Surgical Management and Functional Outcome
Neurosurg Focus 27 (2):E9, 2009 Gliomas of the cingulate gyrus: surgical management and functional outcome MAREC VON LEHE , M.D., AN D JOHANNES SCHRA mm , M.D. Neurochirurgische Klinik, Universitätsklinik Bonn, Germany Object. In this paper, the authors’ goal was to summarize their experience with the surgical treatment of gliomas arising from the cingulate gyrus. Methods. The authors analyzed preoperative data, surgical strategies, complications, and functional outcome in a series of 34 patients (mean age 42 years, range 12–69 years; 14 females) who underwent 38 operations between May 2001 and November 2008. Results. In 7 cases (18%) the tumor was located in the posterior (parietal) part of the cingulate gyrus, and in 31 (82%) the tumor was in the anterior (frontal) part. In 10 cases (26%) the glioma was solely located in the cingulate gyrus, and in 28 cases (74%) the tumor extended to the supracingular frontal/parietal cortex. Most cases (23 [61%]) had seizures as the presenting symptom, 8 patients (24%) suffered from a hemiparesis/hemihypesthesia, and 4 pa- tients (12%) had aphasic symptoms. The authors chose an interhemispheric approach for tumor resection in 11 (29%) and a transcortical approach in 27 (71%) cases; intraoperative electrophysiological monitoring was applied in 23 (61%) and neuronavigation in 15 (39%) cases. A > 90% resection was achieved in 32 (84%) and > 70% in another 5 (13%) cases. Tumors were classified as low-grade gliomas in 11 cases (29%). A glioblastoma multiforme (WHO Grade IV, 10 cases [26%]) and oligoastrocytoma (WHO Grade III, 9 cases [24%]) were the most frequent histopathological results. -

Differentiable Processing of Objects, Associations and Scenes Within the Hippocampus
bioRxiv preprint doi: https://doi.org/10.1101/208827; this version posted October 25, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Differentiable processing of objects, associations and scenes within the hippocampus Marshall A. Dalton, Peter Zeidman, Cornelia McCormick, Eleanor A. Maguire* Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College London, UK Keywords: hippocampus, scene construction, relational, associative, memory, pre/parasubiculum, subfields, fMRI, objects, perirhinal *Corresponding author at: Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College London, 12 Queen Square, London, WC1N 3BG, UK. T: +44-20-34484362; F: +44-20-78131445; E: [email protected] (E.A. Maguire) 1 bioRxiv preprint doi: https://doi.org/10.1101/208827; this version posted October 25, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Highlights . Theories generally posit that the hippocampus (HC) executes one fundamental process . We compared several of these processes in a rigorously matched manner using fMRI . Dissociable processing of objects, associations and scenes was evident in the HC . The HC is not uni-functional and extant theories may need to be revised eTOC blurb Dalton et al. show that there is dissociable processing of objects, associations and scenes within the hippocampus. -

Occipital Sulci Patterns in Patients with Schizophrenia and Migraine Headache Using Magnetic Resonance Imaging (MRI)
ORIGINAL ARTICLE Occipital sulci patterns in patients with schizophrenia and migraine headache using magnetic resonance imaging (MRI) Gorana Sulejmanpašić1, Enra Suljić2, Selma Šabanagić-Hajrić2 1Department of Psychiatry, 2Department of Neurology; University Clinical Center Sarajevo, Sarajevo, Bosnia and Herzegovina ABSTRACT Aim To examine the presence of morphologic variations of occi- pital sulci patternsin patients with schizophrenia and migraine he- adacheregarding gender and laterality using magnetic resonance imaging (MRI). MethodsThis study included 80 patients and brain scans were performed to analyze interhemispheric symmetry and the sulcal patterns of the occipital region of both hemispheres. Average total volumes of both hemispheres of the healthy population were used for comparison. Results There was statistically significant difference between su- bjects considering gender (p=0.012)with no difference regarding Corresponding author: age(p=0.1821). Parameters of parieto-occipital fissure (p=0.0314), Gorana Sulejmanpašić body of the calcarine sulcus (p=0.0213), inferior sagittal sulcus (p=0.0443), and lateral occipital sulcus (p=0.0411) showed stati- Department of Psychiatry, Clinical Center stically significant difference only of left hemisphere in male pati- University of Sarajevo ents with schizophrenia with shallowerdepth of the sulcus. Bolnička 25, 71000 Sarajevo, Bosnia and Herzegovina Conclusion Representation of neuroanatomical structures sugge- sts the existence of structural neuroanatomic disorders with focal Phone: +387 33 29 75 38; brain changes. Comparative analysis of occipital lobe and their Fax:+387 33 29 85 23; morphologic structures (cortical dysmorphology) in patients with E mail: [email protected] schizophreniausing MRI, according to genderindicates a signifi- cant cortical reduction in the left hemisphere only in the group of male patients compared to female patients and the control group. -
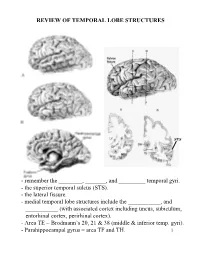
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

Supporting Information for “Endocast Morphology of Homo Naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, S
Supporting Information for “Endocast Morphology of Homo naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, Shawn D. Hurst, Heather M. Garvin, P. Thomas Schoenemann, William B. Vanti, Lee R. Berger, and John Hawks What follows are our descriptions, illustrations, some basic interpretation, and a more specific discussion of the functional, comparative, and taxonomic issues surrounding these hominins. We use the neuroanatomical nomenclature from Duvernoy (19). DH1 Occipital The DH1 occipital fragment (Figs 1, S2) measures ca 105 mm in width between left temporo- occipital incisure and right sigmoid sinus. It is 61 mm in height on the left side, and 47 mm on the right side. The fragment covers the entire left and mostly complete right occipital lobes. The lobes are strongly asymmetrical, with the left clearly larger than the right, and more posteriorly protruding. There are faint traces of a lateral remnant of the lunate sulcus on the left side, and a dorsal bounding lunate as well (#4 and #6 in Fig 1). The right side shows a very small groove at the end of the lateral sinus, which could be a remnant of the lunate sulcus. The major flow from the longitudinal sinus is to the right. Small portions of both cerebellar lobes, roughly 15 mm in height are present. There is a suggestion of a great cerebellar sulcus on the right side. The width from the left lateral lunate impression to the midline is 43mm. The distance from left occipital pole (the most posteriorly projecting point, based on our best estimate of the proper orientation) to the mid-sagittal plane is 30 mm. -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/200480 Please be advised that this information was generated on 2021-10-05 and may be subject to change. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). Extensive discussions of the cerebral sulci and their variations were presented by Ono et al. (1990), Duvernoy (1992), Tamraz and Comair (2000), and Rhoton (2007). An anatomical parcellation of the spatially normalized single high resolution T1 volume provided by the Montreal Neurological Institute (MNI; Collins, 1994; Collins et al., 1998) was used for the macroscopical labeling of functional studies (Tzourio-Mazoyer et al., 2002; Rolls et al., 2015). In the standard atlas of the human brain by Mai et al. (2016), the terminology from Mai and Paxinos (2012) is used. It contains an extensively analyzed individual brain hemisphere in the MNI- space. -

Potential Scalp Stimulation Targets for Mental Disorders
Cao et al. J Transl Med (2021) 19:343 https://doi.org/10.1186/s12967-021-02993-1 Journal of Translational Medicine RESEARCH Open Access Potential scalp stimulation targets for mental disorders: evidence from neuroimaging studies Jin Cao, Thalia Celeste Chai‑Zhang, Yiting Huang, Maya Nicole Eshel and Jian Kong* Abstract Mental disorders widely contribute to the modern global disease burden, creating a signifcant need for improvement of treatments. Scalp stimulation methods (such as scalp acupuncture and transcranial electrical stimulation) have shown promising results in relieving psychiatric symptoms. However, neuroimaging fndings haven’t been well‑ integrated into scalp stimulation treatments. Identifying surface brain regions associated with mental disorders would expand target selection and the potential for these interventions as treatments for mental disorders. In this study, we performed large‑scale meta‑analyses separately on eight common mental disorders: attention defcit hyperactivity disorder, anxiety disorder, autism spectrum disorder, bipolar disorder, compulsive disorder, major depression, post‑ traumatic stress disorder and schizophrenia; utilizing modern neuroimaging literature to summarize disorder‑asso‑ ciated surface brain regions, and proposed neuroimaging‑based target protocols. We found that the medial frontal gyrus, the supplementary motor area, and the dorsal lateral prefrontal cortex are commonly involved in the patho‑ physiology of mental disorders. The target protocols we proposed may provide new brain targets for scalp stimulation in the treatment of mental disorders, and facilitate its clinical application. Keywords: Neuroimaging, Meta‑analysis, Scalp stimulation, Scalp acupuncture, Transcranial electrical stimulation, Mental disorder Introduction For instance, scalp acupuncture, a modern school of Mental disorders are a major component of the modern acupuncture developed on the basis of anatomical and global disease burden.