Acetylcholinesterase Staining in Human Auditory and Language
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -
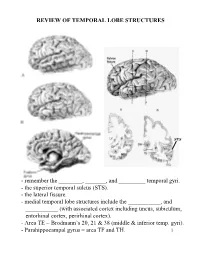
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -

Surgical Anatomy and Techniques
SURGICAL ANATOMY AND TECHNIQUES MICROSURGICAL APPROACHES TO THE MEDIAL TEMPORAL REGION:AN ANATOMICAL STUDY Alvaro Campero, M.D. OBJECTIVE: To describe the surgical anatomy of the anterior, middle, and posterior Department of Neurological Surgery, portions of the medial temporal region and to present an anatomic-based classification University of Florida, of the approaches to this area. Gainesville, Florida METHODS: Twenty formalin-fixed, adult cadaveric specimens were studied. Ten brains Gustavo Tro´ccoli, M.D. provided measurements to compare different surgical strategies. Approaches were demon- Department of Neurological Surgery, strated using 10 silicon-injected cadaveric heads. Surgical cases were used to illustrate the Hospital “Dr. J. Penna,” results by the different approaches. Transverse lines at the level of the inferior choroidal point Bahı´a Blanca, Argentina and quadrigeminal plate were used to divide the medial temporal region into anterior, middle, and posterior portions. Surgical approaches to the medial temporal region were classified into Carolina Martins, M.D. four groups: superior, lateral, basal, and medial, based on the surface of the lobe through which Department of Neurological Surgery, University of Florida, the approach was directed. The approaches through the medial group were subdivided further Gainesville, Florida into an anterior approach, the transsylvian transcisternal approach, and two posterior ap- proaches, the occipital interhemispheric and supracerebellar transtentorial approaches. Juan C. Fernandez-Miranda, M.D. RESULTS: The anterior portion of the medial temporal region can be reached through Department of Neurological Surgery, University of Florida, the superior, lateral, and basal surfaces of the lobe and the anterior variant of the Gainesville, Florida approach through the medial surface. -

The Depth Asymmetry of Superior Temporal Sulcus
New human-specific brain landmark: The depth asymmetry of superior temporal sulcus François Leroya,1, Qing Caib, Stephanie L. Bogartc, Jessica Duboisa, Olivier Coulond, Karla Monzalvoa, Clara Fischere, Hervé Glasela, Lise Van der Haegenf, Audrey Bénézita, Ching-Po Ling, David N. Kennedyh, Aya S. Iharai, Lucie Hertz-Pannierj, Marie-Laure Moutardk, Cyril Pouponl, Marc Brysbaerte, Neil Robertsm, William D. Hopkinsc, Jean-François Mangine, and Ghislaine Dehaene-Lambertza aCognitive Neuroimaging Unit, U992, eAnalysis and Processing of Information Unit, jClinical and Translational Applications Research Unit, U663, and lNuclear Magnetic Resonance Imaging and Spectroscopy Unit, Office of Atomic Energy and Alternative Energies (CEA), INSERM, NeuroSpin, Gif-sur-Yvette 91191, France; bShanghai Key Laboratory of Brain Functional Genomics, East China Normal University, Shanghai 200241, China; cDivision of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA 30322; dLaboratory for Systems and Information Science, UMR CNRS 7296, Aix-Marseille University, Marseille 13284, France; fDepartment of Experimental Psychology, Ghent University, Ghent B-9000, Belgium; gInstitute of Neuroscience, National Yang-Ming University, Taipei City 112, Taiwan; hCenter for Morphometric Analysis, Neuroscience Center, Massachusetts General Hospital, Boston, MA 02114; iCenter for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka 565-0871 Japan; kNeuropediatrics Department, Trousseau -

A Practical Review of Functional MRI Anatomy of the Language and Motor Systems
REVIEW ARTICLE FUNCTIONAL A Practical Review of Functional MRI Anatomy of the Language and Motor Systems X V.B. Hill, X C.Z. Cankurtaran, X B.P. Liu, X T.A. Hijaz, X M. Naidich, X A.J. Nemeth, X J. Gastala, X C. Krumpelman, X E.N. McComb, and X A.W. Korutz ABSTRACT SUMMARY: Functional MR imaging is being performed with increasing frequency in the typical neuroradiology practice; however, many readers of these studies have only a limited knowledge of the functional anatomy of the brain. This text will delineate the locations, anatomic boundaries, and functions of the cortical regions of the brain most commonly encountered in clinical practice—specifically, the regions involved in movement and language. ABBREVIATIONS: FFA ϭ fusiform face area; IPL ϭ inferior parietal lobule; PPC ϭ posterior parietal cortex; SMA ϭ supplementary motor area; VOTC ϭ ventral occipitotemporal cortex his article serves as a review of the functional areas of the brain serving to analyze spatial position and the ventral stream working Tmost commonly mapped during presurgical fMRI studies, to identify what an object is. Influenced by the dorsal and ventral specifically targeting movement and language. We have compiled stream model of vision, Hickok and Poeppel2 hypothesized a sim- what we hope is a useful, easily portable, and concise resource that ilar framework for language. In this model, the ventral stream, or can be accessible to radiologists everywhere. We begin with a re- lexical-semantic system, is involved in sound-to-meaning map- view of the language-processing system. Then we describe the pings associated with language comprehension and semantic ac- gross anatomic boundaries, organization, and function of each cess. -

Seed MNI Coordinates Lobe
MNI Coordinates Seed Lobe (Hemisphere) Region BAa X Y Z FP1 -18 62 0 Frontal Lobe (L) Medial Frontal Gyrus 10 FPz 4 62 0 Frontal Lobe (R) Medial Frontal Gyrus 10 FP2 24 60 0 Frontal Lobe (R) Superior Frontal Gyrus 10 AF7 -38 50 0 Frontal Lobe (L) Middle Frontal Gyrus 10 AF3 -30 50 24 Frontal Lobe (L) Superior Frontal Gyrus 9 AFz 4 58 30 Frontal Lobe (R) Medial Frontal Gyrus 9 AF4 36 48 20 Frontal Lobe (R) Middle Frontal Gyrus 10 AF8 42 46 -4 Frontal Lobe (R) Inferior Frontal Gyrus 10 F7 -48 26 -4 Frontal Lobe (L) Inferior Frontal Gyrus 47 F5 -48 28 18 Frontal Lobe (L) Inferior Frontal Gyrus 45 F3 -38 28 38 Frontal Lobe (L) Precentral Gyrus 9 F1 -20 30 50 Frontal Lobe (L) Superior Frontal Gyrus 8 Fz 2 32 54 Frontal Lobe (L) Superior Frontal Gyrus 8 F2 26 32 48 Frontal Lobe (R) Superior Frontal Gyrus 8 F4 42 30 34 Frontal Lobe (R) Precentral Gyrus 9 F6 50 28 14 Frontal Lobe (R) Middle Frontal Gyrus 46 F8 48 24 -8 Frontal Lobe (R) Inferior Frontal Gyrus 47 FT9 -50 -6 -36 Temporal Lobe (L) Inferior Temporal Gyrus 20 FT7 -54 2 -8 Temporal Lobe (L) Superior Temporal Gyrus 22 FC5 -56 4 22 Frontal Lobe (L) Precentral Gyrus 6 FC3 -44 6 48 Frontal Lobe (L) Middle Frontal Gyrus 6 FC1 -22 6 64 Frontal Lobe (L) Middle Frontal Gyrus 6 FCz 4 6 66 Frontal Lobe (R) Medial Frontal Gyrus 6 FC2 28 8 60 Frontal Lobe (R) Sub-Gyral 6 FC4 48 8 42 Frontal Lobe (R) Middle Frontal Gyrus 6 FC6 58 6 16 Frontal Lobe (R) Inferior Frontal Gyrus 44 FT8 54 2 -12 Temporal Lobe (R) Superior Temporal Gyrus 38 FT10 50 -6 -38 Temporal Lobe (R) Inferior Temporal Gyrus 20 T7/T3 -
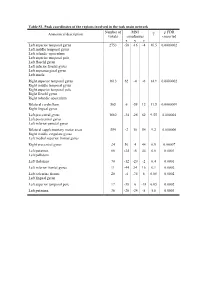
Table S1. Peak Coordinates of the Regions Involved in the Task Main
Table S1. Peak coordinates of the regions involved in the task main network Number of MNI p FDR Anatomical description T voxels coordinates corrected x y z Left superior temporal gyrus 2753 -58 -16 -4 16.5 0.0000002 Left middle temporal gyrus Left rolandic operculum Left superior temporal pole Left Heschl gyrus Left inferior frontal gyrus Left supramarginal gyrus Left insula Right superior temporal gyrus 1613 62 -4 -6 14.9 0.0000002 Right middle temporal gyrus Right superior temporal pole Right Heschl gyrus Right rolandic operculum Bilateral cerebellum 565 6 -58 12 11.5 0.0000009 Right lingual gyrus Left precentral gyrus 1682 -34 -26 62 9.55 0.000004 Left postcentral gyrus Left inferior parietal gyrus Bilateral supplementary motor areas 554 -2 10 54 9.2 0.000006 Right middle cingulate gyrus Left medial superior frontal gyrus Right precentral gyrus 24 56 4 44 6.9 0.00007 Left putamen 66 -24 -6 44 6.6 0.0001 Left pallidum Left thalamus 70 -12 -20 -2 6.4 0.0001 Left inferior frontal gyrus 11 -44 24 16 6.1 0.0002 Left calcarine fissure 20 -4 -74 8 6.08 0.0002 Left lingual gyrus Left superior temporal pole 17 -38 6 -18 6.05 0.0002 Left putamen 36 -28 -24 -8 5.8 0.0003 Table S2. Peak coordinates of the regions showing a main effect of item type Number of MNI p FDR Anatomical description T voxels coordinates corrected x y z Words – Pseudowords Left inferior frontal gyrus 96 -40 24 -6 7.6 0.000001 Left insula Left superior temporal pole Left middle temporal gyrus 141 -56 -36 -2 5.9 0.000015 Left precentral gyrus 26 -50 8 32 4.9 0.00025 Left inferior -

Pars Triangularis Asymmetry and Language Dominance ANNE L
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 719-722, January 1996 Neurobiology Pars triangularis asymmetry and language dominance ANNE L. FOUNDAS*, CHRISTIANA M. LEONARDO§, ROBIN L. GILMOREt¶, EILEEN B. FENNELLtII, AND KENNETH M. HEILMAN4:¶** *Department of Psychiatry and Neurology, Tulane University School of Medicine and Neurology Service, Veterans Affairs Medical Center, 1430 Tulane Avenue, New Orleans, LA 70112-2632; and *Center for Neuropsychological Studies, Departments of §Neuroscience and 1Neurology and I'Clinical and Health Psychology, University of Florida College of Medicine and **Neurology Service, Veterans Affairs Medical Center, Gainesville, FL 32608-1197 Communicated by Philip Teitelbaum, University of Florida, Gainesville, FL, October 10, 1995 (received for review March 15, 1995) ABSTRACT The pars triangularis is a portion of Broca's planum temporale can be performed (19-22). Given these area. The convolutions that form the inferior and caudal advances in MRI technology, reliable measures of language extent of the pars triangularis include the anterior horizontal laterality and handedness can be evaluated in the same indi- and anterior ascending rami of the sylvian fissure, respec- viduals, thus allowing direct structure-function correlations. tively. To learn if there are anatomic asymmetries of the pars Using this technique, Steinmetz et al. (22) studied planum triangularis, these convolutions were measured on volumetric temporale asymmetries in a group of left- and right-handers. magnetic resonance imaging scans of 11 patients who had A leftward asymmetry of the planum temporale was docu- undergone selective hemispheric anesthesia (Wada testing) to mented with a greater degree of leftward asymmetry in the determine hemispheric speech and language lateralization. -

Normal Cortical Anatomy
Normal Cortical Anatomy MGH Massachusetts General Hospital Harvard Medical School NORMAL CORTICAL ANATOMY • Sagittal • Axial • Coronal • The Central Sulcus NP/MGH Sagittal Neuroanatomy NP/MGH Cingulate sulcus Superior frontal gyrus Marginal ramus of Cingulate sulcus Cingulate gyrus Paracentral lobule Superior parietal lobule Parietooccipital sulcus Cuneus Calcarine sulcus Lingual gyrus Subcallosal gyrus Gyrus rectus Fastigium, fourth ventricle NP/MGH Superior frontal gyrus Cingulate sulcus Precentral gyrus Marginal ramus of Cingulate gyrus Central sulcus Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Cuneus Calcarine sulcus Frontomarginal gyrus Lingual gyrus Caudothallamic groove Gyrus rectus NP/MGH Precentral sulcus Central sulcus Superior frontal gyrus Marginal ramus of Corona radiata Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Calcarine sulcus Inferior occipital gyrus Lingual gyrus NP/MGH Central sulcus Superior parietal lobule Parietooccipital sulcus Frontopolar gyrus Frontomarginal gyrus Superior occipital gyrus Middle occipital gyrus Medial orbital gyrus Lingual gyrus Posterior orbital gyrus Inferior occipital gyrus Inferior temporal gyrus Temporal horn, lateral ventricle NP/MGH Central sulcus Superior Temporal gyrus Middle Temporal gyrus Inferior Temporal gyrus NP/MGH Central sulcus Superior parietal gyrus Inferior frontal gyrus Frontomarginal gyrus Anterior orbital gyrus Superior occipital gyrus Middle occipital Posterior orbital gyrus gyrus Superior Temporal gyrus Inferior -
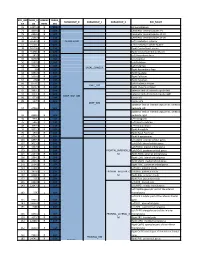
Core-Example1.Pdf
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital