Sex-Specific Differences in Resting-State Functional Connectivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

Acetylcholinesterase Staining in Human Auditory and Language
Acetylcholinesterase Staining in Human Jeffrey J. Hutsler and Michael S. Gazzaniga Auditory and Language Cortices: Center for Neuroscience, University of California, Davis Regional Variation of Structural Features California 95616 Cholinergic innovation of the cerebral neocortex arises from the basal 1974; Greenfield, 1984, 1991; Robertson, 1987; Taylor et al., forebrain and projects to all cortical regions. Acetylcholinesterase 1987; Krisst, 1989; Small, 1989, 1990). AChE-containing axons (AChE), the enzyme responsible for deactivating acetylcholine, is found of the cerebral cortex are also immunoreactive for choline within both cholinergic axons arising from the basal forebrain and a acetyltransferase (ChAT) and are therefore known to be cho- Downloaded from subgroup of pyramidal cells in layers III and V of the cerebral cortex. linergic (Mesulam and Geula, 1992). This pattern of staining varies with cortical location and may contrib- AChE-containing pyramidal cells of layers III and V are not ute uniquely to cortical microcircuhry within functionally distinct cholinergic (Mesulam and Geula, 1991), but it has been sug- regions. To explore this issue further, we examined the pattern of AChE gested that they are cholinoceptive (Krnjevic and Silver, 1965; staining within auditory, auditory association, and putative language Levey et al., 1984; Mesulam et al., 1984b). In support of this regions of whole, postmortem human brains. notion, layer in and V pyramidal cell excitability can be mod- http://cercor.oxfordjournals.org/ The density and distribution of acetylcholine-containing axons and ulated by the application of acetylcholine in the slice prepa- pyramidal cells vary systematically as a function of auditory process- ration (McCormick and Williamson, 1989). Additionally, mus- ing level. -

Resting-State Fmri Reveals Network Disintegration During Delirium T ⁎ Simone J.T
NeuroImage: Clinical 20 (2018) 35–41 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Resting-state fMRI reveals network disintegration during delirium T ⁎ Simone J.T. van Montforta, , Edwin van Dellenb,c, Aletta M.R. van den Boscha,d, Willem M. Ottee,f, Maya J.L. Schutteb, Soo-Hee Choig, Tae-Sub Chungh, Sunghyon Kyeongi, Arjen J.C. Slootera, ⁎⁎ Jae-Jin Kimi, a Department of Intensive Care Medicine, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht University, The Netherlands b Department of Psychiatry, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht University, the Netherlands c Melbourne Neuropsychiatry Center, Department of Psychiatry, University of Melbourne, Australia d Faculty of Science, University of Amsterdam, the Netherlands e Biomedical MR Imaging and Spectroscopy Group, Center for Image Sciences, University Medical Center Utrecht, Utrecht University, the Netherlands f Department of Pediatric Neurology, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht University, the Netherlands g Department of Psychiatry, Institute of Human Behavioral Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea h Department of Radiology, Yonsei University Gangnam Severance Hospital, Seoul, Republic of Korea i Department of Psychiatry, Institute of Behavioral Science in Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea. ARTICLE INFO ABSTRACT Keywords: Delirium is characterized by inattention and other cognitive deficits, symptoms that have been associated with Delirium disturbed interactions between remote brain regions. Recent EEG studies confirm that disturbed global network fMRI topology may underlie the syndrome, but lack an anatomical basis. The aim of this study was to increase our Resting-state understanding of the global organization of functional connectivity during delirium and to localize possible Brain networks alterations. -
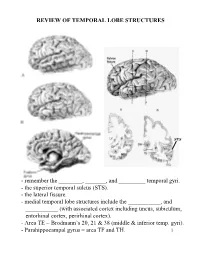
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

Electroencephalographic Resting-State Functional Connectivity of Benign Epilepsy with Centrotemporal Spikes
JCN Open Access ORIGINAL ARTICLE pISSN 1738-6586 / eISSN 2005-5013 / J Clin Neurol 2019;15(2):211-220 / https://doi.org/10.3988/jcn.2019.15.2.211 Electroencephalographic Resting-State Functional Connectivity of Benign Epilepsy with Centrotemporal Spikes Hyun-Soo Choia* Background and Purpose We aimed to reveal resting-state functional connectivity char- Yoon Gi Chungb* c acteristics based on the spike-free waking electroencephalogram (EEG) of benign epilepsy Sun Ah Choi with centrotemporal spikes (BECTS) patients, which usually appears normal in routine visual d Soyeon Ahn inspection. c Hunmin Kim Methods Thirty BECTS patients and 30 disease-free and age- and sex-matched controls Sungroh Yoona were included. Eight-second EEG epochs without artifacts were sampled and then bandpass Hee Hwangc filtered into the delta, theta, lower alpha, upper alpha, and beta bands to construct the associa- Ki Joong Kime,f tion matrix. The weighted phase lag index (wPLI) was used as an association measure for EEG signals. The band-specific connectivity, which was represented as a matrix of wPLI values of a Department of Electrical and all edges, was compared for analyzing the connectivity itself. The global wPLI, characteristic Computer Engineering, path length (CPL), and mean clustering coefficient were compared. Seoul National University, Seoul, Korea Results The resting-state functional connectivity itself and the network topology differed in bHealthcare ICT Research Center, the BECTS patients. For the lower-alpha-band and beta-band connectivity, edges that showed Seoul National University significant differences had consistently lower wPLI values compared to the disease-free con- Bundang Hospital, Seongnam, Korea c trols. -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -

Time-Resolved Resting-State Brain Networks
Time-resolved resting-state brain networks Andrew Zaleskya,b,1, Alex Fornitoa,c, Luca Cocchid, Leonardo L. Golloe, and Michael Breakspeare,f,g aMelbourne Neuropsychiatry Centre, The University of Melbourne and Melbourne Health, Melbourne, VIC 3010, Australia; bMelbourne School of Engineering, The University of Melbourne, Melbourne, VIC 3010, Australia; cMonash Clinical and Imaging Neuroscience, School of Psychological Sciences and Monash Biomedical Imaging, Monash University, Melbourne, VIC 3168, Australia; dQueensland Brain Institute, The University of Queensland, Brisbane, QLD 4072, Australia; and eQIMR Berghofer Medical Research Institute, fThe Royal Brisbane and Women’s Hospital, and gMetro North Mental Health, Brisbane, QLD 4029, Australia Edited by Michael S. Gazzaniga, University of California, Santa Barbara, CA, and approved May 19, 2014 (received for review January 13, 2014) Neuronal dynamics display a complex spatiotemporal structure consistently revealed fluctuations in resting-state functional con- involving the precise, context-dependent coordination of acti- nectivity at timescales ranging from tens of seconds to a few vation patterns across a large number of spatially distributed minutes (19–24). Furthermore, the modular organization of func- regions. Functional magnetic resonance imaging (fMRI) has played tional brain networks appears to be time-dependent in the resting a central role in demonstrating the nontrivial spatial and topolog- state (25, 26) and modulated by learning (27) and cognitive effort ical structure of these interactions, but thus far has been limited in (28, 29). It is therefore apparent that reducing fluctuations in its capacity to study their temporal evolution. Here, using high- functional connectivity to time averages has led to a very useful but ’ resolution resting-state fMRI data obtained from the Human static and possibly oversimplified characterization of the brain s Connectome Project, we mapped time-resolved functional connec- functional networks. -

Psychedelic Resting-State Neuroimaging: a Review and Perspective on Balancing Replication and Novel Analyses
Psychedelic resting-state neuroimaging: a review and perspective on balancing replication and novel analyses Drummond E-Wen McCulloch1, Gitte Moos Knudsen1,2, Frederick Streeter Barrett3, Manoj Doss3, Robin Lester Carhart-Harris4,5, Fernando E. Rosas5,6,7, Gustavo Deco8,9,10,11,12, Katrin H. Preller13, Johannes G. Ramaekers14, Natasha L. Mason14, Felix Müller15, Patrick MacDonald Fisher1 1Neurobiology Research Unit, Rigshospitalet, 2100 Copenhagen, Denmark 2Institute of Clinical Medicine, University of Copenhagen, 2100 Copenhagen, Denmark 3Department of Psychiatry and Behavioral Sciences, Center for Psychedelic and Consciousness Research, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA 4Neuroscape, Weill Institute for Neurosciences, University of California San Francisco, USA 5Centre for Psychedelic Research, Department of Brain Sciences, Imperial College London, London SW7 2DD, UK 6Data Science Institute, Imperial College London, London SW7 2AZ, UK 7Centre for Complexity Science, Imperial College London, London SW7 2AZ, UK 8Center for Brain and Cognition, Computational Neuroscience Group, Universitat Pompeu Fabra, Barcelona, Spain, 9Department of Information and Communication Technologies, Universitat Pompeu Fabra, Barcelona, Spain, 10Institució Catalana de la Recerca i Estudis Avançats (ICREA), Barcelona, Spain, 11Department of Neuropsychology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 12School of Psychological Sciences, Monash University, Melbourne, Australia 13Pharmaco-Neuroimaging -

Surgical Anatomy and Techniques
SURGICAL ANATOMY AND TECHNIQUES MICROSURGICAL APPROACHES TO THE MEDIAL TEMPORAL REGION:AN ANATOMICAL STUDY Alvaro Campero, M.D. OBJECTIVE: To describe the surgical anatomy of the anterior, middle, and posterior Department of Neurological Surgery, portions of the medial temporal region and to present an anatomic-based classification University of Florida, of the approaches to this area. Gainesville, Florida METHODS: Twenty formalin-fixed, adult cadaveric specimens were studied. Ten brains Gustavo Tro´ccoli, M.D. provided measurements to compare different surgical strategies. Approaches were demon- Department of Neurological Surgery, strated using 10 silicon-injected cadaveric heads. Surgical cases were used to illustrate the Hospital “Dr. J. Penna,” results by the different approaches. Transverse lines at the level of the inferior choroidal point Bahı´a Blanca, Argentina and quadrigeminal plate were used to divide the medial temporal region into anterior, middle, and posterior portions. Surgical approaches to the medial temporal region were classified into Carolina Martins, M.D. four groups: superior, lateral, basal, and medial, based on the surface of the lobe through which Department of Neurological Surgery, University of Florida, the approach was directed. The approaches through the medial group were subdivided further Gainesville, Florida into an anterior approach, the transsylvian transcisternal approach, and two posterior ap- proaches, the occipital interhemispheric and supracerebellar transtentorial approaches. Juan C. Fernandez-Miranda, M.D. RESULTS: The anterior portion of the medial temporal region can be reached through Department of Neurological Surgery, University of Florida, the superior, lateral, and basal surfaces of the lobe and the anterior variant of the Gainesville, Florida approach through the medial surface. -

The Depth Asymmetry of Superior Temporal Sulcus
New human-specific brain landmark: The depth asymmetry of superior temporal sulcus François Leroya,1, Qing Caib, Stephanie L. Bogartc, Jessica Duboisa, Olivier Coulond, Karla Monzalvoa, Clara Fischere, Hervé Glasela, Lise Van der Haegenf, Audrey Bénézita, Ching-Po Ling, David N. Kennedyh, Aya S. Iharai, Lucie Hertz-Pannierj, Marie-Laure Moutardk, Cyril Pouponl, Marc Brysbaerte, Neil Robertsm, William D. Hopkinsc, Jean-François Mangine, and Ghislaine Dehaene-Lambertza aCognitive Neuroimaging Unit, U992, eAnalysis and Processing of Information Unit, jClinical and Translational Applications Research Unit, U663, and lNuclear Magnetic Resonance Imaging and Spectroscopy Unit, Office of Atomic Energy and Alternative Energies (CEA), INSERM, NeuroSpin, Gif-sur-Yvette 91191, France; bShanghai Key Laboratory of Brain Functional Genomics, East China Normal University, Shanghai 200241, China; cDivision of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA 30322; dLaboratory for Systems and Information Science, UMR CNRS 7296, Aix-Marseille University, Marseille 13284, France; fDepartment of Experimental Psychology, Ghent University, Ghent B-9000, Belgium; gInstitute of Neuroscience, National Yang-Ming University, Taipei City 112, Taiwan; hCenter for Morphometric Analysis, Neuroscience Center, Massachusetts General Hospital, Boston, MA 02114; iCenter for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka 565-0871 Japan; kNeuropediatrics Department, Trousseau -

A Practical Review of Functional MRI Anatomy of the Language and Motor Systems
REVIEW ARTICLE FUNCTIONAL A Practical Review of Functional MRI Anatomy of the Language and Motor Systems X V.B. Hill, X C.Z. Cankurtaran, X B.P. Liu, X T.A. Hijaz, X M. Naidich, X A.J. Nemeth, X J. Gastala, X C. Krumpelman, X E.N. McComb, and X A.W. Korutz ABSTRACT SUMMARY: Functional MR imaging is being performed with increasing frequency in the typical neuroradiology practice; however, many readers of these studies have only a limited knowledge of the functional anatomy of the brain. This text will delineate the locations, anatomic boundaries, and functions of the cortical regions of the brain most commonly encountered in clinical practice—specifically, the regions involved in movement and language. ABBREVIATIONS: FFA ϭ fusiform face area; IPL ϭ inferior parietal lobule; PPC ϭ posterior parietal cortex; SMA ϭ supplementary motor area; VOTC ϭ ventral occipitotemporal cortex his article serves as a review of the functional areas of the brain serving to analyze spatial position and the ventral stream working Tmost commonly mapped during presurgical fMRI studies, to identify what an object is. Influenced by the dorsal and ventral specifically targeting movement and language. We have compiled stream model of vision, Hickok and Poeppel2 hypothesized a sim- what we hope is a useful, easily portable, and concise resource that ilar framework for language. In this model, the ventral stream, or can be accessible to radiologists everywhere. We begin with a re- lexical-semantic system, is involved in sound-to-meaning map- view of the language-processing system. Then we describe the pings associated with language comprehension and semantic ac- gross anatomic boundaries, organization, and function of each cess.