Surgical Anatomy and Techniques
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

A Pictorial Essay on Anatomy and Pathology of the Hippocampus
Insights Imaging DOI 10.1007/s13244-016-0541-2 PICTORIAL REVIEW BUnforgettable^ – a pictorial essay on anatomy and pathology of the hippocampus Sven Dekeyzer 1,2,3 & Isabelle De Kock2 & Omid Nikoubashman1 & Stephanie Vanden Bossche 2 & Ruth Van Eetvelde2,3 & Jeroen De Groote2 & Marjan Acou2 & Martin Wiesmann1 & Karel Deblaere2 & Eric Achten2 Received: 19 September 2016 /Revised: 18 December 2016 /Accepted: 20 December 2016 # The Author(s) 2017. This article is published with open access at Springerlink.com Abstract • Clinical information is often necessary to come to a correct The hippocampus is a small but complex anatomical structure diagnosis or an apt differential. that plays an important role in spatial and episodic memory. The hippocampus can be affected by a wide range of congen- Keywords Hippocampus . Epilepsy . Dementia . Herpes ital variants and degenerative, inflammatory, vascular, tumoral simplex encephalitis . MRI and toxic-metabolic pathologies. Magnetic resonance imaging is the preferred imaging technique for evaluating the hippo- campus. The main indications requiring tailored imaging se- Abbreviations quences of the hippocampus are medically refractory epilepsy AD Alzheimer’sdementia and dementia. The purpose of this pictorial review is three- DNET Dysembryoblastic neuroepithelial tumour fold: (1) to review the normal anatomy of the hippocampus on IHI Incomplete hippocampal inversion MRI; (2) to discuss the optimal imaging strategy for the eval- HSE Herpes simplex encephalitis uation of the hippocampus; and (3) to present a pictorial over- LE Limbic encephalitis view of the most common anatomic variants and pathologic MTA Mesial temporal atrophy conditions affecting the hippocampus. MTS Mesial temporal sclerosis Teaching points • Knowledge of normal hippocampal anatomy helps recognize Anatomy, embryology, arterial supply and function anatomic variants and hippocampal pathology. -

Multi-Contrast Submillimetric 3Tesla Hippocampal Subfield Segmentation
www.nature.com/scientificdata OPEN Multi-contrast submillimetric SUBJECT CATEGORIES » Brain imaging 3Tesla hippocampal subfield » Magnetic resonance imaging segmentation protocol and dataset » Brain Jessie Kulaga-Yoskovitz1,*, Boris C. Bernhardt1,2,*, Seok-Jun Hong1, Tommaso Mansi3, » Neurology Kevin E. Liang1, Andre J.W. van der Kouwe4, Jonathan Smallwood5, Andrea Bernasconi1,* » Neuroscience & Neda Bernasconi1,* The hippocampus is composed of distinct anatomical subregions that participate in multiple cognitive processes and are differentially affected in prevalent neurological and psychiatric conditions. Advances in high-field MRI allow for the non-invasive identification of hippocampal substructure. These approaches, however, demand time-consuming manual segmentation that relies heavily on anatomical expertise. Here, Received: 10 August 2015 we share manual labels and associated high-resolution MRI data (MNI-HISUB25; submillimetric T1- and Accepted: 07 October 2015 T2-weighted images, detailed sequence information, and stereotaxic probabilistic anatomical maps) based Published: 10 November 2015 on 25 healthy subjects. Data were acquired on a widely available 3 Tesla MRI system using a 32 phased- array head coil. The protocol divided the hippocampal formation into three subregions: subicular complex, merged Cornu Ammonis 1, 2 and 3 (CA1-3) subfields, and CA4-dentate gyrus (CA4-DG). Segmentation was guided by consistent intensity and morphology characteristics of the densely myelinated molecular layer together with few geometry-based boundaries -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Acetylcholinesterase Staining in Human Auditory and Language
Acetylcholinesterase Staining in Human Jeffrey J. Hutsler and Michael S. Gazzaniga Auditory and Language Cortices: Center for Neuroscience, University of California, Davis Regional Variation of Structural Features California 95616 Cholinergic innovation of the cerebral neocortex arises from the basal 1974; Greenfield, 1984, 1991; Robertson, 1987; Taylor et al., forebrain and projects to all cortical regions. Acetylcholinesterase 1987; Krisst, 1989; Small, 1989, 1990). AChE-containing axons (AChE), the enzyme responsible for deactivating acetylcholine, is found of the cerebral cortex are also immunoreactive for choline within both cholinergic axons arising from the basal forebrain and a acetyltransferase (ChAT) and are therefore known to be cho- Downloaded from subgroup of pyramidal cells in layers III and V of the cerebral cortex. linergic (Mesulam and Geula, 1992). This pattern of staining varies with cortical location and may contrib- AChE-containing pyramidal cells of layers III and V are not ute uniquely to cortical microcircuhry within functionally distinct cholinergic (Mesulam and Geula, 1991), but it has been sug- regions. To explore this issue further, we examined the pattern of AChE gested that they are cholinoceptive (Krnjevic and Silver, 1965; staining within auditory, auditory association, and putative language Levey et al., 1984; Mesulam et al., 1984b). In support of this regions of whole, postmortem human brains. notion, layer in and V pyramidal cell excitability can be mod- http://cercor.oxfordjournals.org/ The density and distribution of acetylcholine-containing axons and ulated by the application of acetylcholine in the slice prepa- pyramidal cells vary systematically as a function of auditory process- ration (McCormick and Williamson, 1989). Additionally, mus- ing level. -

Human Parietal Cortex in Action Jody C Culham and Kenneth F Valyear
Human parietal cortex in action Jody C Culham and Kenneth F Valyear Experiments using functional neuroimaging and transcranial owes, in large part, to the rapid growth of neuroimaging magnetic stimulation in humans have revealed regions of the studies, particularly experiments using functional mag- parietal lobes that are specialized for particular visuomotor netic resonance imaging (fMRI) and transcranial mag- actions, such as reaching, grasping and eye movements. In netic stimulation (TMS). addition, the human parietal cortex is recruited by processing and perception of action-related information, even when no In one popular view of the visual system [1], visual overt action occurs. Such information can include object shape information is segregated along two pathways: the ventral and orientation, knowledge about how tools are employed and stream (occipito-temporal cortex) computes vision for the understanding of actions made by other individuals. We perception, whereas the dorsal stream (occipito-parietal review the known subregions of the human posterior parietal cortex) computes vision for action. Here, we review cortex and the principles behind their organization. recent advances that address the organization of the posterior parietal cortex and the action-related subregions Addresses within it. We begin by focusing on the role of the dorsal Department of Psychology, Social Science Centre, University of stream in visually-guided real actions. However, we then Western Ontario, London, Ontario, Canada, N6A 5C2 discuss a topic that -

Neuroanatomy: Dissection of the Sheep Brain
Neuroanatomy: Dissection of the Sheep Brain The basic neuroanatomy of the mammalian brain is similar for all species. Instead of using a rodent brain (too small) or a human brain (no volunteer donors), we will study neuroanatomy by examining the brain of the sheep. We will be looking as several structures within the central nervous system (CNS), which is composed of the brain and spinal cord. You will learn individual structures now; later you will be looking at brain systems to see how different parts of the brain form functional units. Just as we differ with respect to individual characteristics, brains also differ slightly in the size, coloration, and shape of some structures. Carefully examine the brain you and your group are dissecting and try to look around the room at some other brains so you can see how the structures may vary. This is especially important when examining the cranial nerves, since rarely will all 12 nerve pairs be preserved on any one brain. This handout will guide you through the dissection and identification of structures. Refer to your text and the accompanying CD for additional information about comparative structures and functions. The last page of this handout lists all the structures you should be prepared to identify for the test. If the structure is marked with *, you should be prepared to identify the function of that structure. Check out the terrific tutorial of a sheep brain dissection on the web site at the University of Scranton (http://academic.uofs.edu/department/psych/sheep/). Lateral View of the Sheep Brain Dorsal Horizontal Section Anterior Posterior Ventral Olfactory bulbs Optic nerves Look for Medulla and chiasm pituitary here External and Midline Anatomy Examine the sheep brain with the membranes intact. -
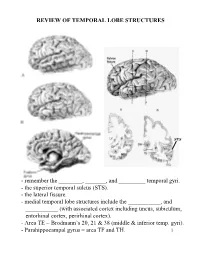
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

Acetylcholinesterase Fiber Staining in the Human Hippocampus and Parahippocampal Gyrms
THE JOURNAL OF COMPARATIVE NEUROLOGY 273:488-499 (1988) Acetylcholinesterase Fiber Staining in the Human Hippocampus and Parahippocampal Gyrms ROBERT C. GREEN AND M-MARSEL MESULAM Division of Neuroscience and Behavioral Neurology, Beth Israel Hospital and Harvard Medical School, Boston, Massachusetts 02215 ABSTRACT The AChE fiber distribution within the human hippocampus and para- hippocampal gyrus was studied in order to provide normative data for the examination of cholinergic fiberarchitecture in human pathology and to clarify the cytoarchitectonic organization of these structures. A modification of the Koelle method was used to stain temporal lobe serial sections from 6 neurologically normal human brains collected at autopsy. The hippocampal formation contains some ofthe densest staining of any cortical area. Regions with the heaviest concentrations of AChE fibers in- clude a thin band along the inner edge of the molecular layer of the dentate gyrus (ml-DG) and parts of the CA2, CA3, and CA4 sectors of Ammon's horn. Staining is of intermediate intensity in the CA1 region, The subiculum (S) is more lightly stained than the CA fields. Staining in the parahippocam- pal gyrus is generally less dense than in the hippocampal formation. The most conspicuous feature of the human entorhinal cortex (EC) is the AChE- rich fiber patches seen overlapping the stellate cell islands in layer 11. An additional band of relatively dense AChE staining is identified in layers N- V. Prominent AChE-rich polymorphic neurons are present within the hilum of the dentate gyrus. The CAlhubiculum transition in Nissl preparation is characterized by an oblique interdigitation of CA1 cells. The transition from EC to prorhinal cortex occurs along the medial bank of the rhinal sulcus and is characterized by a band of AChE staining, which slopes obliquely away from layer I1 until it joins an intermediate pyramidal cell layer. -

Differential Projection from the Motor and Limbic Cortical Regions to the Mediodorsal Thalamic Nucleus in the Dog
ACTA NEUROBIOL. EXP. 1989. 49: 23-37 DIFFERENTIAL PROJECTION FROM THE MOTOR AND LIMBIC CORTICAL REGIONS TO THE MEDIODORSAL THALAMIC NUCLEUS IN THE DOG Iwona STEPNIEWSKA and Anna KOSMAL Department of Neurophysiology, Nencki Institute of Experimental Biology 3 Pasteur Str., 02-093 Warsaw, Poland Key words: mediodorsal thalamic nucleus, motor and limbic cortices, horseradish peroxidase Abstract. The cortical afferents to the mediodorsal thalamic nucleus in the dog were studied by using horseradish peroxidase. Small injec- tions allowed to establish two specific projection zones connected sepa- rately with the lateral and medial segments of the nucleus. The lateral segment received the major projection from the dorsal half of the hemi- sphere. It included premotor and part of the motor cortices in the ante- rior sigmoid gyrus and precruciate areas as well as the presylvian cor- tex. The medial segment of the nucleus was innervated by the limbic areas of the ventral half of the hemisphere. These areas included the medloventrally located genual, subcallosal and plriform cortices, as well as the cortex of the ventral bank of the anterior rhinal sulcus and the caudal part of the orbital gyrus. The cortical fields situated between these two main cortical zones, both on the lateral and medial surfaces (rhinal and sylvian sulci and anterior cingular gyrus, respectively) sent projec- tions to both medial and lateral segments of the nucleus. These results indicate that in the mediodorsal thalamic nucleus may take place the integration of information from two functionally defined systems, the motor and limbic ones. INTRODUCTIOPJ The numerous studies of the cortical afferents of the mediodorsal thalamic nucleus (MD) showed, that although the prefrontal cortex (PFC) gives rise to the heaviest projection to MD, it is not the only cortical region projecting to thls nucleus. -

Dissection Technique for the Study of the Cerebral Sulci, Gyri and Ventricles
Arq Neuropsiquiatr 2008;66(2-A):282-287 Dissection technique for the stuDy of the cerebral sulci, gyri anD ventricles João Paulo Mattos1, Marcos Juliano dos Santos2, João Flavio Daniel Zullo2, Andrei Fernandes Joaquim2, Feres Chaddad-Neto1, Evandro de Oliveira3 Abstract – Neuroanatomy in addition to neurophysiology, are the basic areas for the proper formation from health students to specialized professionals in neuroscience. A step by step guide for practical studies of neuroanatomy is required for this kind of knowledge to become more acceptable among medical students, neurosurgeons, neurologists, neuropediatricians and psychiatric physicians. Based on the well known courses of sulci, gyri and ventricles offered by Beneficência Portuguesa Hospital in São Paulo, Brazil, two times a year, since 1994, totalizing more than 20 complete courses, and answering the request of many neuroscience students and professionals whose asked for a practical guide to the neuroanatomy study, the authors suggest a protocol for the study of superficial and deep brain structures showing how to approach the more structures as possible with minimum damage to the anatomic piece and with the smaller number of brains. Key wordS: neuroanatomy, brain, dissection technique. técnica de dissecação para o estudo dos sulcos, giros e ventriculos cerebrais Resumo – Neuroanatomia e a neurofisiologia são as áreas básicas para a adequada formação desde estudantes na área da saúde a profissionais especializados em neurociências. Um guia prático, passo a passo, para o estudo