Interaction of Inferior Temporal Cortex with Frontal Cortex and Basal Forebrain: Double Dissociation in Strategy Implementation and Associative Learning
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Neuronal Activity in Monkey Ventral Striatum Related to the Expectation of Reward
The Journal of Neuroscience. December 1992. 72(12): 45954610 Neuronal Activity in Monkey Ventral Striatum Related to the Expectation of Reward Wolfram Schultz, Paul Apicella,” Eugenio Scarnati,b and Tomas Ljungbergc lnstitut de Physiologie, Universitk de Fribourg, CH-1700 Fribourg, Switzerland Projections from cortical and subcortical limbic structures The anatomical substrate underlying these functions may con- to the basal ganglia are predominantly directed to the ventral sist in the conjunction of afferents from limbic structures and striatum. The present study investigated how the expecta- mesencephalicdopamine neurons. Major limbic structures in tion of external events with behavioral significance is re- monkeys, such asthe anterior cingulate gyms, orbitofrontal cor- flected in the activity of ventral striatal neurons. A total of tex, and amygdala, project to the ventral striatum, including the 420 neurons were studied in macaque monkeys performing nucleus accumbens,in a particularly dense and interdigitating in a delayed go-no-go task. Lights of different colors in- fashion, whereas their projections to the dorsal striatum are structed the animal to do an arm-reaching movement or re- more sparseand scattered(Baleydier and Mauguiere, 1980; Par- frain from moving, respectively, when a trigger light was ent et al., 1983; Russchenet al., 1985; Selemonand Goldman- illuminated a few seconds later. Task performance was re- Rakic, 1985). The amygdala is involved in the association of inforced by liquid reward in both situations. A total of 60 external stimuli with primary and secondaryreinforcers for sus- ventral striatal neurons showed sustained increases of ac- taining performance in learning tasks (Gaffan and Harrison, tivity before the occurrence of individual task events. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Audiovisual Integration of Letters in the Human Brain
Neuron, Vol. 28, 617±625, November, 2000, Copyright 2000 by Cell Press Audiovisual Integration of Letters in the Human Brain Tommi Raij,* Kimmo Uutela, and Riitta Hari dalities to allow them then to interact. Therefore, brain Brain Research Unit areas participating in, e.g., audiovisual integration would Low Temperature Laboratory be expected to show signs of (1) convergence (both Helsinki University of Technology auditory and visual stimuli should activate the same P.O. Box 2200 region) and (2) interaction (the activation evoked by au- FIN-02015-HUT diovisual stimulation should differ from the sum of uni- Espoo modally presented auditory and visual activations). Finland Our aim was to study the human brain's audiovisual integration mechanisms for letters, i.e., for stimuli that have been previously associated through learning. For Summary literate people, the alphabet is effortlessly transformed between the auditory and visual domains (and transmit- Letters of the alphabet have auditory (phonemic) and ted to the motor systems for speech and writing). Our visual (graphemic) qualities. To investigate the neural subjects received auditory, visual, and audiovisual let- representations of such audiovisual objects, we re- ters of the roman alphabet and were required to identify corded neuromagnetic cortical responses to audi- them, regardless of stimulus modality. Audiovisual let- torily, visually, and audiovisually presented single let- ters included matching letters, in which the auditory ters. The auditory and visual brain activations first and visual stimulus corresponded to each other based converged around 225 ms after stimulus onset and on previous experience, and nonmatching (randomly then interacted predominantly in the right temporo- paired) letters. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -
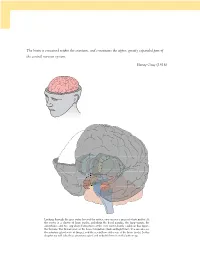
The Brain Is Contained Within the Cranium, and Constitutes the Upper, Greatly Expanded Part of the Central Nervous System
The brain is contained within the cranium, and constitutes the upper, greatly expanded part of the central nervous system. Henry Gray (1918) Looking through the gray outer layer of the cortex, you can see a mass of white matter. At the center is a cluster of large nuclei, including the basal ganglia, the hippocampi, the amygdalae, and two egg-shaped structures at the very center, barely visible in this figure, the thalami. The thalami rest on the lower brainstem (dark and light blue). You can also see the pituitary gland in front (beige), and the cerebellum at the rear of the brain (pink). In this chapter we will take these structures apart and re-build them from the bottom up. 09_P375070_Ch05.indd 126 1/29/2010 4:08:25 AM CHAPTER 5 The brain OUTLINE 1.0 Introduction 127 3.2 Output and input: the front-back division 143 1.1 The nervous system 128 3.3 The major lobes: visible and hidden 145 1.2 The geography of the brain 129 3.4 The massive interconnectivity of the cortex and thalamus 149 2.0 Growing a brain from the bottom up 133 3.5 The satellites of the subcortex 151 2.1 Evolution and personal history are expressed in the brain 133 4.0 Summary 153 2.2 Building a brain from bottom to top 134 5.0 Chapter review 153 3.0 From ‘ where ’ to ‘ what ’ : the functional 5.1 Study questions 153 roles of brain regions 136 5.2 Drawing exercises 153 3.1 The cerebral hemispheres: the left-right division 136 1.0 INTRODUCTION found. -

Boosting Learning Efficacy with Non-Invasive Brain Stimulation in Intact and Brain-Damaged Humans
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Behavioral/Cognitive Boosting learning efficacy with non-invasive brain stimulation in intact and brain-damaged humans F. Herpich1,2,3, F. Melnick, M.D.4 , S. Agosta1 , K.R. Huxlin4 , D. Tadin4 and L Battelli1,5,6 1Center for Neuroscience and Cognitive Systems@UniTn, Istituto Italiano di Tecnologia, Corso Bettini 31, 38068 Rovereto (TN), Italy 2Center for Mind/Brain Sciences, University of Trento, 38068 Rovereto, Italy 3kbo Klinikum-Inn-Salzach, Gabersee 7, 83512, Wasserburg am Inn, Germany 4Department of Brain and Cognitive Sciences, Flaum Eye Institute and Center for Visual Science, University of Rochester, Rochester, NY, USA 5Berenson-Allen Center for Noninvasive Brain Stimulation and Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, 02215 Massachusetts, USA 6Cognitive Neuropsychology Laboratory, Harvard University, Cambridge, MA, USA https://doi.org/10.1523/JNEUROSCI.3248-18.2019 Received: 28 December 2018 Revised: 10 April 2019 Accepted: 8 May 2019 Published: 27 May 2019 Author contributions: F.H., M.D.M., K.H., D.T., and L.B. designed research; F.H., M.D.M., and S.A. performed research; F.H., M.D.M., and D.T. analyzed data; F.H., M.D.M., and L.B. wrote the first draft of the paper; M.D.M., S.A., K.H., D.T., and L.B. edited the paper; K.H., D.T., and L.B. wrote the paper. Conflict of Interest: The authors declare no competing financial interests. The present study was funded by the Autonomous Province of Trento, Call “Grandi Progetti 2012”, project “Characterizing and improving brain mechanisms of attention — ATTEND (FH, SA, LB), “Fondazione Caritro — Bando Ricerca e Sviluppo Economico” (FH), NIH (DT, MM and KRH: R01 grants EY027314 and EY021209, CVS training grant T32 EY007125), and by an unrestricted grant from the Research to Prevent Blindness (RPB) Foundation to the Flaum Eye Institute. -

AN ANALYSIS of RIGHT- and LEFT-BRAIN THINKERS and CERTAIN STYLES of LEARNING by Steven D. Bielefeldt a Research Paper Submitted
AN ANALYSIS OF RIGHT- AND LEFT-BRAIN THINKERS AND CERTAIN STYLES OF LEARNING BY Steven D. Bielefeldt A Research Paper Submitted in Partial Fulfillment of the Requirements for the Master of Science Degree Education Approved: 2 Semester Credits Ax I Dr. Robert Sedlak The Graduate School University of Wisconsin -Stout May 2006 The Graduate School University of Wisconsin -Stout Menomonie, WI Author: Bielefeldt, Steven D. Title: An Analysis of Right- and Left-Brain Thinkers and Certain Styles of Learning Graduate Degree I Major: MS Education Research Advisor: Robert Sedlak, Ph.D. Month I Year: May, 2006 Number of Pages: 29 Style Manual Used: American Psychological Association, sth edition ABSTRACT The purpose of this study was to analyze right- and left- brain thinkers and certain styles of learning (specifically visual, auditory, or kinesthetic) in college- level adult learners. This study includes data collected from approximately 100 adult learners with a survey, as well as a comprehensive review and analysis of literature concerning the brain, brain-based learning, and certain learning styles. Research-based evidence from the study will be used to ellcourage instructors to develop and use appropriate styles of teaching to enhance their student's educational experience. Table of Contents ABSTRACT ........................................................................................................... ii List of Tables ......................................................................................................... V Chapter I: -

Neural Plasticity Underlying Visual Perceptual Learning in Aging
brain research ] ( ]]]]) ]]]– ]]] Available online at www.sciencedirect.com www.elsevier.com/locate/brainres Research Report Neural plasticity underlying visual perceptual learning in aging Jyoti Mishraa,n, Camarin Rollea, Adam Gazzaleya,b,c,nn aDepartment of Neurology, University of California, San Francisco, San Francisco, CA, USA bDepartment of Physiology, University of California, San Francisco, San Francisco, CA, USA cDepartment of Psychiatry, University of California, San Francisco, San Francisco, CA, USA article info abstract Article history: Healthy aging is associated with a decline in basic perceptual abilities, as well as higher-level Accepted 2 September 2014 cognitive functions such as working memory. In a recent perceptual training study using moving sweeps of Gabor stimuli, Berry et al. (2010) observed that older adults significantly Keywords: improved discrimination abilities on the most challenging perceptual tasks that presented Aging paired sweeps at rapid rates of 5 and 10 Hz. Berry et al. further showed that this perceptual fi Visual perception training engendered transfer-of-bene t to an untrained working memory task. Here, we Cognitive training investigated the neural underpinnings of the improvements in these perceptual tasks, as Perceptual learning assessed by event-related potential (ERP) recordings. Early visual ERP components time- Working memory locked to stimulus onset were compared pre- and post-training, as well as relative to a no- fi Transfer of benefit contact control group. The visual N1 and N2 components were signi cantly enhanced after ERP training, and the N1 change correlated with improvements in perceptual discrimination on the task. Further, the change observed for the N1 and N2 was associated with the rapidity of the perceptual challenge; the visual N1 (120–150 ms) was enhanced post-training for 10 Hz sweep pairs, while the N2 (240–280 ms) was enhanced for the 5 Hz sweep pairs. -

Reading Speech from Still and Moving Faces: the Neural Substrates of Visible Speech
Reading Speech from Still and Moving Faces: The Neural Substrates of Visible Speech Gemma A. Calvert1 and Ruth Campbell2 Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/15/1/57/1757731/089892903321107828.pdf by guest on 18 May 2021 Abstract & Speech is perceived both by ear and by eye. Unlike heard regions including the left inferior frontal (Broca’s) area, left speech, some seen speech gestures can be captured in superior temporal sulcus (STS), and left supramarginal gyrus stilled image sequences. Previous studies have shown that in (the dorsal aspect of Wernicke’s area). Stilled speech hearing people, natural time-varying silent seen speech can sequences also generated activation in the ventral premotor access the auditory cortex (left superior temporal regions). cortex and anterior inferior parietal sulcus bilaterally. Using functional magnetic resonance imaging (fMRI), the Moving faces generated significantly greater cortical activa- present study explored the extent to which this circuitry was tion than stilled face sequences, and in similar regions. activated when seen speech was deprived of its time-varying However, a number of differences between stilled and moving characteristics. speech were also observed. In the visual cortex, stilled faces In the scanner, hearing participants were instructed to generated relatively more activation in primary visual regions look for a prespecified visible speech target sequence (‘‘voo’’ (V1/V2), while visual movement areas (V5/MT+) were or ‘‘ahv’’) among other monosyllables. In one condition, the activated to a greater extent by moving faces. Cortical regions image sequence comprised a series of stilled key frames activated more by naturally moving speaking faces included showing apical gestures (e.g., separate frames for ‘‘v’’ and the auditory cortex (Brodmann’s Areas 41/42; lateral parts of ‘‘oo’’ [from the target] or ‘‘ee’’ and ‘‘m’’ [i.e., from Heschl’s gyrus) and the left STS and inferior frontal gyrus. -

The Visual Corticostriatal Loop Through the Tail of the Caudate: Circuitry and Function
HYPOTHESIS AND THEORY ARTICLE published: 06 December 2013 SYSTEMS NEUROSCIENCE doi: 10.3389/fnsys.2013.00104 The visual corticostriatal loop through the tail of the caudate: circuitry and function CarolA.Seger* Program in Molecular, Cellular, and Integrative Neuroscience, Department of Psychology, Colorado State University, Fort Collins, CO, USA Edited by: Although high level visual cortex projects to a specific region of the striatum, the tail of the Ahmed A. Moustafa, University of caudate, and participates in corticostriatal loops, the function of this visual corticostriatal Western Sydney, Australia system is not well understood. This article first reviews what is known about the anatomy Reviewed by: of the visual corticostriatal loop across mammals, including rodents, cats, monkeys, and Erick J. Paul, University of Illinois Urbana Champaign, USA humans. Like other corticostriatal systems, the visual corticostriatal system includes Greg Ashby, University of California, both closed loop components (recurrent projections that return to the originating cortical Santa Barbara, USA location) and open loop components (projections that terminate in other neural regions). *Correspondence: The article then reviews what previous empirical research has shown about the function Carol A. Seger, Program in of the tail of the caudate. The article finally addresses the possible functions of the closed Molecular, Cellular, and Integrative Neuroscience, Department of and open loop connections of the visual loop in the context of theories and computational Psychology, Colorado State models of corticostriatal function. University, Mail code 1876, Fort Collins, CO 80523, USA Keywords: striatum, caudate, category learning, basal ganglia, corticostriatal, recurrent neural network, e-mail: [email protected] reinforcement learning, Area TE INTRODUCTION forming both open and closed loops. -

Windows to the Brain: Introduction to Neuroanatomy
VA Mid-Atlantic Health Care Network Windows to the Brain: Introduction to Neuroanatomy Overview Planes of Section Radiographic Perspective Major Divisions Cortical Lobes, Gyri & Sulci General Functions Brodmann’s Areas Basal Forebrain Subcortical Structures Symptoms Cerebellum Structures Symptoms Katherine Taber, PhD, FANPA MIRECC Assistant Director - Education Research Health Scientist W.G. “Bill” Hefner VAMC, Salisbury NC Research Professor, Div Biomedical Sciences Edward Via College of Osteopathic Medicine Robin Hurley, MD, FANPA MIRECC Associate Director - Education ACOS/Research and Academic Affairs Service Line W.G. “Bill” Hefner VAMC, Salisbury NC Professor, Psychiatry and Radiology Wake Forest School of Medicine revised September 2019 Source: http://www.mirecc.va.gov/visn6/Tools-Tips.asp Use of text, images and other content are subject to the following terms and conditions: Fair Use Is Permitted Fair use of copyrighted material includes the use for non-commercial educational purposes, such as teaching, scholarship, research, criticism, commentary, news reporting, and other content. Unless otherwise noted, users who wish to download or print text and image files from this Web site for such uses may do so without the VISN 6 MIRECC’s express permission, provided that they comply with the following conditions: The content may only be used for personal, educational or non- commercial purposes; Users must always specifically cite the author(s) and source of the content every time the material is used, as they would for material from any printed work; None of the content may be altered or modified. Warranty By downloading, printing, or otherwise using text and image files from this website, users agree and warrant that they will limit their use of such files to fair use. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger).