Pars Triangularis Asymmetry and Language Dominance ANNE L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Network Architecture of the Cerebral Nuclei (Basal Ganglia) Association and Commissural Connectome
Network architecture of the cerebral nuclei (basal ganglia) association and commissural connectome Larry W. Swansona,1, Olaf Spornsb, and Joel D. Hahna aDepartment of Biological Sciences, University of Southern California, Los Angeles, CA 90089; and bDepartment of Psychological and Brain Sciences, Indiana University, Bloomington, IN 47405 Contributed by Larry W. Swanson, August 10, 2016 (sent for review July 18, 2016; reviewed by Ann M. Graybiel and Liqun Luo) The cerebral nuclei form the ventral division of the cerebral hemi- same basic strategy and methodology applied to the rat cerebral sphere and are thought to play an important role in neural systems cortical association macroconnectome (10) but with additional an- controlling somatic movement and motivation. Network analysis alytical approaches and curation tools. In this approach a macro- was used to define global architectural features of intrinsic cerebral connection is defined as a monosynaptic axonal (directed, from/to) nuclei circuitry in one hemisphere (association connections) and connection between two nervous system gray matter regions or between hemispheres (commissural connections). The analysis was between a gray matter region and another part of the body (such as based on more than 4,000 reports of histologically defined axonal a muscle) (11, 12). All 45 gray matter regions of the cerebral nuclei connections involving all 45 gray matter regions of the rat cerebral on each side of the brain were included in the analysis. The goal of nuclei and revealed the existence of four asymmetrically intercon- this analysis was to provide global, high-level, design principles of nected modules. The modules form four topographically distinct intrinsic cerebral nuclei circuitry as a framework for more detailed research at the meso-, micro-, and nanolevels of analysis (13). -

Gyrification from Constrained Cortical Expansion
Gyrification from constrained cortical expansion Tuomas Tallinena, Jun Young Chungb, John S. Bigginsc, and L. Mahadevand,e,f,1 aDepartment of Physics and Nanoscience Center, University of Jyväskylä, FI-40014 Jyväskylä, Finland; bSchool of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138; cCavendish Laboratory, Cambridge University, Cambridge CB3 0HE, United Kingdom; dWyss Institute for Biologically Inspired Engineering, eKavli Institute for Bionano Science and Technology, and fSchool of Engineering and Applied Sciences, Department of Organismic and Evolutionary Biology, and Department of Physics, Harvard University, Cambridge, MA 02138 Edited* by John W. Hutchinson, Harvard University, Cambridge, MA, and approved July 15, 2014 (received for review April 1, 2014) The exterior of the mammalian brain—the cerebral cortex—has from an unregulated and unpatterned growth of the cortex rel- a conserved layered structure whose thickness varies little across ative to sublayers. species. However, selection pressures over evolutionary time scales Nevertheless, there is as yet no explicit biologically and physi- have led to cortices that have a large surface area to volume ratio in cally plausible model that can convincingly reproduce individual some organisms, with the result that the brain is strongly convo- sulci and gyri, let alone the complex patterns of sulci and gyri luted into sulci and gyri. Here we show that the gyrification can found in the brain. Early attempts to mechanically model brain arise as a nonlinear consequence of a simple mechanical instability folding (13) were rooted in the physics of wrinkling and assumed driven by tangential expansion of the gray matter constrained by a thin stiff layer of gray matter that grows relative to a thick soft the white matter. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Basic Brain Anatomy
Chapter 2 Basic Brain Anatomy Where this icon appears, visit The Brain http://go.jblearning.com/ManascoCWS to view the corresponding video. The average weight of an adult human brain is about 3 pounds. That is about the weight of a single small To understand how a part of the brain is disordered by cantaloupe or six grapefruits. If a human brain was damage or disease, speech-language pathologists must placed on a tray, it would look like a pretty unim- first know a few facts about the anatomy of the brain pressive mass of gray lumpy tissue (Luria, 1973). In in general and how a normal and healthy brain func- fact, for most of history the brain was thought to be tions. Readers can use the anatomy presented here as an utterly useless piece of flesh housed in the skull. a reference, review, and jumping off point to under- The Egyptians believed that the heart was the seat standing the consequences of damage to the structures of human intelligence, and as such, the brain was discussed. This chapter begins with the big picture promptly removed during mummification. In his and works down into the specifics of brain anatomy. essay On Sleep and Sleeplessness, Aristotle argued that the brain is a complex cooling mechanism for our bodies that works primarily to help cool and The Central Nervous condense water vapors rising in our bodies (Aristo- tle, republished 2011). He also established a strong System argument in this same essay for why infants should not drink wine. The basis for this argument was that The nervous system is divided into two major sec- infants already have Central nervous tions: the central nervous system and the peripheral too much moisture system The brain and nervous system. -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Congenital Microcephaly
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sussex Research Online American Journal of Medical Genetics Part C (Seminars in Medical Genetics) ARTICLE Congenital Microcephaly DIANA ALCANTARA AND MARK O'DRISCOLL* The underlying etiologies of genetic congenital microcephaly are complex and multifactorial. Recently, with the exponential growth in the identification and characterization of novel genetic causes of congenital microcephaly, there has been a consolidation and emergence of certain themes concerning underlying pathomechanisms. These include abnormal mitotic microtubule spindle structure, numerical and structural abnormalities of the centrosome, altered cilia function, impaired DNA repair, DNA Damage Response signaling and DNA replication, along with attenuated cell cycle checkpoint proficiency. Many of these processes are highly interconnected. Interestingly, a defect in a gene whose encoded protein has a canonical function in one of these processes can often have multiple impacts at the cellular level involving several of these pathways. Here, we overview the key pathomechanistic themes underlying profound congenital microcephaly, and emphasize their interconnected nature. © 2014 Wiley Periodicals, Inc. KEY WORDS: cell division; mitosis; DNA replication; cilia How to cite this article: Alcantara D, O'Driscoll M. 2014. Congenital microcephaly. Am J Med Genet Part C Semin Med Genet 9999:1–16. INTRODUCTION mid‐gestation although glial cell division formation of the various cortical layers. and consequent brain volume enlarge- Furthermore, differentiating and devel- Congenital microcephaly, an occipital‐ ment does continue after birth [Spalding oping neurons must migrate to their frontal circumference of equal to or less et al., 2005]. Impaired neurogenesis is defined locations to construct the com- than 2–3 standard deviations below the therefore most obviously reflected clini- plex architecture and laminar layered age‐related population mean, denotes cally as congenital microcephaly. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Acetylcholinesterase Staining in Human Auditory and Language
Acetylcholinesterase Staining in Human Jeffrey J. Hutsler and Michael S. Gazzaniga Auditory and Language Cortices: Center for Neuroscience, University of California, Davis Regional Variation of Structural Features California 95616 Cholinergic innovation of the cerebral neocortex arises from the basal 1974; Greenfield, 1984, 1991; Robertson, 1987; Taylor et al., forebrain and projects to all cortical regions. Acetylcholinesterase 1987; Krisst, 1989; Small, 1989, 1990). AChE-containing axons (AChE), the enzyme responsible for deactivating acetylcholine, is found of the cerebral cortex are also immunoreactive for choline within both cholinergic axons arising from the basal forebrain and a acetyltransferase (ChAT) and are therefore known to be cho- Downloaded from subgroup of pyramidal cells in layers III and V of the cerebral cortex. linergic (Mesulam and Geula, 1992). This pattern of staining varies with cortical location and may contrib- AChE-containing pyramidal cells of layers III and V are not ute uniquely to cortical microcircuhry within functionally distinct cholinergic (Mesulam and Geula, 1991), but it has been sug- regions. To explore this issue further, we examined the pattern of AChE gested that they are cholinoceptive (Krnjevic and Silver, 1965; staining within auditory, auditory association, and putative language Levey et al., 1984; Mesulam et al., 1984b). In support of this regions of whole, postmortem human brains. notion, layer in and V pyramidal cell excitability can be mod- http://cercor.oxfordjournals.org/ The density and distribution of acetylcholine-containing axons and ulated by the application of acetylcholine in the slice prepa- pyramidal cells vary systematically as a function of auditory process- ration (McCormick and Williamson, 1989). Additionally, mus- ing level. -
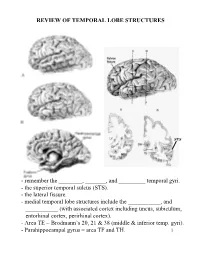
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

The Hominoid-Specific Gene TBC1D3 Promotes Generation of Basal Neural
1 The hominoid-specific gene TBC1D3 promotes generation of basal neural 2 progenitors and induces cortical folding in mice 3 4 Xiang-Chun Ju1,3,9, Qiong-Qiong Hou1,3,9, Ai-Li Sheng1, Kong-Yan Wu1, Yang Zhou8, 5 Ying Jin8, Tieqiao Wen7, Zhengang Yang6, Xiaoqun Wang2,5, Zhen-Ge Luo1,2,3,4 6 7 1Institute of Neuroscience, State Key Laboratory of Neuroscience, Shanghai Institutes for 8 Biological Sciences, Chinese Academy of Sciences, Shanghai, China. 9 2CAS Center for Excellence in Brain Science and Intelligence Technology, Shanghai, 10 China. 11 3Chinese Academy of Sciences University, Beijing, China. 12 4ShanghaiTech University, Shanghai, China 13 5Institute of Biophysics, Chinese Academy of Sciences, Beijing, China. 14 6Institutes of Brain Science, State Key Laboratory of Medical Neurobiology, Fudan 15 University, Shanghai, China. 16 7School of Life Sciences, Shanghai University, Shanghai, China. 17 8The Institute of Health Sciences, Shanghai Institutes for Biological Sciences, Chinese 18 Academy of Sciences, Shanghai, China. 19 9Co-first author 20 Correspondence should be addressed to Z.G.L ([email protected]) 21 22 Author contributions 23 X.-C.J and Q.-Q. H performed most experiments, analyzed data and wrote the paper. 24 A.-L.S helped with in situ hybridization. K.-Y.W assisted with imaging analysis. T.W 25 helped with the construction of nestin promoter construct. Y. Z and Y. J helped with 26 ReNeuron cell culture and analysis. Z.Y provided human fetal samples and assisted with 1 27 immunohistochemistry analysis. X.W provided help with live-imaging analysis. Z.-G.L 28 supervised the whole study, designed the research, analyzed data and wrote the paper. -

Microsurgical and Tractographic Anatomical Study of Insular and Transsylvian Transinsular Approach
Neurol Sci (2011) 32:865–874 DOI 10.1007/s10072-011-0721-2 ORIGINAL ARTICLE Microsurgical and tractographic anatomical study of insular and transsylvian transinsular approach Feng Wang • Tao Sun • XinGang Li • HeChun Xia • ZongZheng Li Received: 29 September 2008 / Accepted: 16 July 2011 / Published online: 24 August 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract This study is to define the operative anatomy of Introduction the insula with emphasis on the transsylvian transinsular approach. The anatomy was studied in 15 brain specimens, In humans, the insula is a highly developed structure, among five were dissected by use of fiber dissection totally encased within the brain. In many clinical and technique; diffusion tensor imaging of 10 healthy volun- experimental studies, a variety of functions have been teers was obtained with a 1.5-T MR system. The temporal attributed to the insula, however, the full and comprehen- stem consists mainly of the uncinate fasciculus, inferior sive role that it plays continues to remain obscure. Oper- occipitofrontal fasciculus, Meyer’s loop of the optic radi- ation of neurosurgery, specifically of epilepsy surgery, is a ation and anterior commissure. The transinsular approach window onto function and dysfunction of the human brain requires an incision of the inferior limiting sulcus. In this [1]. The insula, as part of the paralimbic system, has both procedure, the fibers of the temporal stem can be inter- invasive anatomical and functional connections with the rupted to various degrees. The fiber dissection technique is temporal lobe through white matter fibers [2–6]. -

From Genes to Folds: a Review of Cortical Gyrification Theory
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Brain Struct Funct (2015) 220:2475–2483 DOI 10.1007/s00429-014-0961-z REVIEW From genes to folds: a review of cortical gyrification theory Lisa Ronan • Paul C. Fletcher Received: 5 September 2014 / Accepted: 6 December 2014 / Published online: 16 December 2014 Ó The Author(s) 2014. This article is published with open access at Springerlink.com Abstract Cortical gyrification is not a random process. Introduction: key characteristics of gyrification Instead, the folds that develop are synonymous with the functional organization of the cortex, and form patterns A wing would be a most mystifying structure if one that are remarkably consistent across individuals and even did not know that birds flew. One might observe that some species. How this happens is not well understood. it could be extended a considerable distance that it Although many developmental features and evolutionary had a smooth covering of feathers with conspicuous adaptations have been proposed as the primary cause of markings, that it was operated by powerful muscles, gyrencephaly, it is not evident that gyrification is reducible and that strength and lightness were prominent fea- in this way. In recent years, we have greatly increased our tures of its construction. These are important facts, understanding of the multiple factors that influence cortical but by themselves they do not tell us that birds fly. folding, from the action of genes in health and disease to Yet without knowing this, and without understanding evolutionary adaptations that characterize distinctions something of the principles of flight, a more detailed between gyrencephalic and lissencephalic cortices.