Network Architecture of the Cerebral Nuclei (Basal Ganglia) Association and Commissural Connectome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basic Brain Anatomy
Chapter 2 Basic Brain Anatomy Where this icon appears, visit The Brain http://go.jblearning.com/ManascoCWS to view the corresponding video. The average weight of an adult human brain is about 3 pounds. That is about the weight of a single small To understand how a part of the brain is disordered by cantaloupe or six grapefruits. If a human brain was damage or disease, speech-language pathologists must placed on a tray, it would look like a pretty unim- first know a few facts about the anatomy of the brain pressive mass of gray lumpy tissue (Luria, 1973). In in general and how a normal and healthy brain func- fact, for most of history the brain was thought to be tions. Readers can use the anatomy presented here as an utterly useless piece of flesh housed in the skull. a reference, review, and jumping off point to under- The Egyptians believed that the heart was the seat standing the consequences of damage to the structures of human intelligence, and as such, the brain was discussed. This chapter begins with the big picture promptly removed during mummification. In his and works down into the specifics of brain anatomy. essay On Sleep and Sleeplessness, Aristotle argued that the brain is a complex cooling mechanism for our bodies that works primarily to help cool and The Central Nervous condense water vapors rising in our bodies (Aristo- tle, republished 2011). He also established a strong System argument in this same essay for why infants should not drink wine. The basis for this argument was that The nervous system is divided into two major sec- infants already have Central nervous tions: the central nervous system and the peripheral too much moisture system The brain and nervous system. -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Microsurgical and Tractographic Anatomical Study of Insular and Transsylvian Transinsular Approach
Neurol Sci (2011) 32:865–874 DOI 10.1007/s10072-011-0721-2 ORIGINAL ARTICLE Microsurgical and tractographic anatomical study of insular and transsylvian transinsular approach Feng Wang • Tao Sun • XinGang Li • HeChun Xia • ZongZheng Li Received: 29 September 2008 / Accepted: 16 July 2011 / Published online: 24 August 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract This study is to define the operative anatomy of Introduction the insula with emphasis on the transsylvian transinsular approach. The anatomy was studied in 15 brain specimens, In humans, the insula is a highly developed structure, among five were dissected by use of fiber dissection totally encased within the brain. In many clinical and technique; diffusion tensor imaging of 10 healthy volun- experimental studies, a variety of functions have been teers was obtained with a 1.5-T MR system. The temporal attributed to the insula, however, the full and comprehen- stem consists mainly of the uncinate fasciculus, inferior sive role that it plays continues to remain obscure. Oper- occipitofrontal fasciculus, Meyer’s loop of the optic radi- ation of neurosurgery, specifically of epilepsy surgery, is a ation and anterior commissure. The transinsular approach window onto function and dysfunction of the human brain requires an incision of the inferior limiting sulcus. In this [1]. The insula, as part of the paralimbic system, has both procedure, the fibers of the temporal stem can be inter- invasive anatomical and functional connections with the rupted to various degrees. The fiber dissection technique is temporal lobe through white matter fibers [2–6]. -

A Practical Review of Functional MRI Anatomy of the Language and Motor Systems
REVIEW ARTICLE FUNCTIONAL A Practical Review of Functional MRI Anatomy of the Language and Motor Systems X V.B. Hill, X C.Z. Cankurtaran, X B.P. Liu, X T.A. Hijaz, X M. Naidich, X A.J. Nemeth, X J. Gastala, X C. Krumpelman, X E.N. McComb, and X A.W. Korutz ABSTRACT SUMMARY: Functional MR imaging is being performed with increasing frequency in the typical neuroradiology practice; however, many readers of these studies have only a limited knowledge of the functional anatomy of the brain. This text will delineate the locations, anatomic boundaries, and functions of the cortical regions of the brain most commonly encountered in clinical practice—specifically, the regions involved in movement and language. ABBREVIATIONS: FFA ϭ fusiform face area; IPL ϭ inferior parietal lobule; PPC ϭ posterior parietal cortex; SMA ϭ supplementary motor area; VOTC ϭ ventral occipitotemporal cortex his article serves as a review of the functional areas of the brain serving to analyze spatial position and the ventral stream working Tmost commonly mapped during presurgical fMRI studies, to identify what an object is. Influenced by the dorsal and ventral specifically targeting movement and language. We have compiled stream model of vision, Hickok and Poeppel2 hypothesized a sim- what we hope is a useful, easily portable, and concise resource that ilar framework for language. In this model, the ventral stream, or can be accessible to radiologists everywhere. We begin with a re- lexical-semantic system, is involved in sound-to-meaning map- view of the language-processing system. Then we describe the pings associated with language comprehension and semantic ac- gross anatomic boundaries, organization, and function of each cess. -

Anatomy of Cerebral Hemispheres Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of Cerebral Hemispheres Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: List the parts of the cerebral hemisphere (cortex, medulla, basal nuclei, lateral ventricle). Describe the subdivision of a cerebral hemisphere into lobes. List the important sulci and gyri of each lobe. Describe different types of fibers in cerebral medulla (association, projection and commissural) and give example of each type. Cerebrum Extra Corpus callosum o Largest part of the forebrain. ( makes up 2 / 3 rd weight off all brain) (recall: the forebrain gives the cerebral hemispheres and the diencephalon) o Divided into two halves, the cerebral hemispheres (right and left), which are separated Left hemisphere Right hemisphere by a deep median longitudinal fissure which lodges the falx cerebri*. o In the depth of the fissure, the hemispheres are connected by a bundle of fibers called the corpus callosum. *It is a large, crescent- shaped fold of meningeal layer of dura Median longitudinal fissure mater that descends vertically in the longitudinal fissure between the cerebral Extra Extra hemispheres Cerebrum Buried within the white matter Cerebral Hemispheres lie a number of nuclear masses The structure of cerebral hemipheres includes: (caudate, putamen, globus pallidus) collectively known as the basal ganglia. WM Deeper to the cortex, axons running to and from the cells of the cortex form an extensive mass of white matter (WM). Contains synapses (50 trillion) WM Superficial layer of grey matter, the cerebral cortex. -

IMJ-21-451-En.Pdf
Original Investigation/Orijinal Araştırma İstanbul Med J 2020; 21(6): 451-456 DO I: 10.4274/imj.galenos.2020.09633 Microsurgical and Functional Linguistic Anatomy of Cerebral Basal Ganglia Serebral Bazal Ganglionların Mikrocerrahi Anatomisi ve Dil Üretimi ile İlişkisi Mustafa Güdük1, Musa Çırak2, Baran Bozkurt3, Kaan Yağmurlu3 1Acıbadem Mehmet Ali Aydınlar University, School of Medicine, Department of Neurosurgery, İstanbul, Turkey 2University of Health Sciences Turkey, Bakırköy Dr. Sadi Konuk Training and Research Hospital, Clinic of Neurosurgery, İstanbul, Turkey 3Virginia University, Department of Neurosurgery, Charlottesville, USA ABSTRACT ÖZ Introduction: The central core of the cerebral hemispheres is Amaç: Serebral hemisferlerin derin santral bölgesi; bazal located on the medial side of the insular cortex. It is made ganglionlar (subkortikal gri maddeler) ve kompleks ak madde up of basal ganglia and white matter tracts. The basal ganglia liflerinden oluşur ve insular korteksin hemen mediyalinde yer and their white matter connections serve important motor, alır. Bazal ganglionlar sahip olduğu ak madde lif bağlantıları sensorial, psychological, endocrinological and cognitive sayesinde motor ve sensöriyal, duygu, endokrin düzenleme, functions. Insular gliomas and other deeply located lesions can kognisyon gibi fonksiyonlarda önemli rol oynar. Özellikle cause severe morbidity by affecting the basal ganglia and their insular gliomalar ve derin yerleşimli lezyonlara bağlı, connections. Hence, a thorough understanding of the anatomy bazal ganglionların ve bağlantılarının zarar görmesi ciddi of that area is needed for surgical planning on the insular area. morbiditeye sebep olur. Bu nedenle bu bölgenin mikrocerrahi Methods: We dissected and photographed the insular cortex anatomisinin iyi bilinmesi, insuler bölgeye yapılacak cerrahinin and basal ganglia in five human cadavers via white matter planlanmasında ve cerrahi stratejide çok büyük öneme sahiptir. -

Pars Triangularis Asymmetry and Language Dominance ANNE L
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 719-722, January 1996 Neurobiology Pars triangularis asymmetry and language dominance ANNE L. FOUNDAS*, CHRISTIANA M. LEONARDO§, ROBIN L. GILMOREt¶, EILEEN B. FENNELLtII, AND KENNETH M. HEILMAN4:¶** *Department of Psychiatry and Neurology, Tulane University School of Medicine and Neurology Service, Veterans Affairs Medical Center, 1430 Tulane Avenue, New Orleans, LA 70112-2632; and *Center for Neuropsychological Studies, Departments of §Neuroscience and 1Neurology and I'Clinical and Health Psychology, University of Florida College of Medicine and **Neurology Service, Veterans Affairs Medical Center, Gainesville, FL 32608-1197 Communicated by Philip Teitelbaum, University of Florida, Gainesville, FL, October 10, 1995 (received for review March 15, 1995) ABSTRACT The pars triangularis is a portion of Broca's planum temporale can be performed (19-22). Given these area. The convolutions that form the inferior and caudal advances in MRI technology, reliable measures of language extent of the pars triangularis include the anterior horizontal laterality and handedness can be evaluated in the same indi- and anterior ascending rami of the sylvian fissure, respec- viduals, thus allowing direct structure-function correlations. tively. To learn if there are anatomic asymmetries of the pars Using this technique, Steinmetz et al. (22) studied planum triangularis, these convolutions were measured on volumetric temporale asymmetries in a group of left- and right-handers. magnetic resonance imaging scans of 11 patients who had A leftward asymmetry of the planum temporale was docu- undergone selective hemispheric anesthesia (Wada testing) to mented with a greater degree of leftward asymmetry in the determine hemispheric speech and language lateralization. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
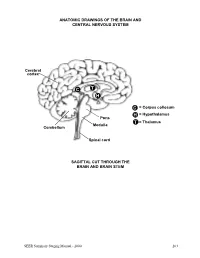
Brain and Central Nervous System
ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM Cerebral cortex C T H C = Corpus collosum H = Hypothalamus Pons T = Thalamus Medulla Cerebellum Spinal cord SAGITTAL CUT THROUGH THE BRAIN AND BRAIN STEM SEER Summary Staging Manual - 2000 263 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM 2 1 3 4 7 5 8 6 SAGITTAL CUT THROUGH THE HUMAN HEAD WITH CEREBRUM IN PLACE The cerebrum is comprised of the: 1 Frontal lobe 2 Parietal lobe 3 Temporal lobe 4 Occipital lobe Other parts of the brain include: 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) 264 SEER Summary Staging Manual - 2000 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM A B C D E 7 5 6 8 F SAGITTAL CUT THROUGH THE HUMAN HEAD Internal anatomy of the brain: A Inner surface of right hemisphere of cerebrum B Corpus callosum C Velum interpositum D Middle commissure E Third ventricle F Fourth ventricle Other parts of the brain (as on previous drawing): 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) SEER Summary Staging Manual - 2000 265 BRAIN AND CEREBRAL MENINGES C70.0, C71.0-C71.9 Supratentorial (S) or Infratentorial (I) C70.0 Cerebral meninges C71.0 Cerebrum ? (S) C71.1 Frontal lobe (S) C71.2 Temporal lobe (S) C71.3 Parietal lobe (S) C71.4 Occipital lobe (S) C71.5 Ventricle, NOS (S) C71.6 Cerebellum, NOS (I) C71.7 Brain stem (I) C71.8 Overlapping lesion of brain ? C71.9 Brain, NOS ? ?See Note 1. SUMMARY STAGE 1 Localized only Supratentorial tumor confined to: Cerebral hemisphere (cerebrum) or meninges of cerebral hemisphere -

Nervous System Pt 3
Write this down… Homework 2 Study Guide (Synapses) Due at the beginning of lab this week Front and back TASS M&W 1-2pm Willamette Hall 204 Thought Question… When you have one of your mandibular teeth worked on at the dentist and he gives you a shot to deaden half of your mouth, what division of the nervous system is being affected by the lidocaine? What do you think it’s mode of action is? Hint: Remember Physio-EX in lab? Is it affecting a cranial or spinal nerve? The Nervous System THE CENTRAL NERVOUS SYSTEM, T H E B R A I N Introduction Integration Memory Learning Sensation and perception Neural Tissue - Definitions White matter versus Gray matter Fiber Bundles Nerves versus Tracts Nerve Cell Bodies Nucleus versus Ganglion White and Gray Matter Central cavity surrounded by a gray matter core External white matter composed of myelinated fiber tracts Brain has additional areas of gray matter not present in spinal cord Central cavity Cortex of gray matter Migratory Inner gray pattern of matter neurons Cerebrum Outer white Cerebellum matter Gray matter Region of cerebellum Central cavity Inner gray matter Outer white matter Gray matter Brain stem Central cavity Outer white matter Inner gray matter Spinal cord Copyright © 2010 Pearson Education, Inc. Figure 12.4 Brain Similar pattern with additional areas of gray matter The Brain Conscious perception Internal regulation Average adult male 3.5 lbs Same brain mass Average adult female 3.2 lbs to body mass ratio! Brain Regions 4 Adult brain regions 1. Cerebral hemispheres (cerebrum) 2. -

Anatomic Moment Limbic System Anatomy: an Overview
Anatomic Moment Limbic System Anatomy: An Overview Leighton P. Mark,1.3 David L. Daniels, 1 Thomas P. Naidich,2 and Jessica A. Borne1 The anatomy of the limbic system has become shaped sulcus (Fig. 2) designated as 1) the hip more relevant due to the development of high pocampal fissure where it lies superior to the resolution and functional magnetic resonance parahippocampal gyrus, and 2) the callosal sulcus (MR) imaging. This review is the first of a series where it lies inferior to the cingulate gyrus of Anatomic Moments designed to highlight se (Fig. 3). lected features of limbic system anatomy in order Nested within this )-shaped sulcus is another ) to facilitate their application to MR interpretation. shaped structure formed by 1) the dentate gyrus Limbic system terminology of early anatomists and hippocampus in the temporal lobe (Figs. 2 was largely descriptive (Table 1), but the nomen and 4), 2) the hippocampal tail which consists of clature used in this series will follow that of recent thin gray and white matter structures located just authors (Table 2) (1-7). posterior and inferior to the splenium of the In accordance with the curvilinear development corpus callosum (Fig. 5), and 3) the supracallosal of the cerebral hemispheres (Fig. 1), the struc gyrus which is located inferior to the callosal tures of the limbic lobe form a series of "nested" sulcus. The supracallosal gyrus is intimately ap )-shaped curves (Fig. 2). plied to the upper surface of the corpus callosum, The widest curve is formed by the large limbic and contains gray matter termed the indusium gyrus which is designated as 1) the parahippo griseum (Fig.