Improving a Probabilistic Cytoarchitectonic Atlas of Auditory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

'What' but Not 'Where' Auditory Processing Pathway
NeuroImage 82 (2013) 295–305 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Emotion modulates activity in the ‘what’ but not ‘where’ auditory processing pathway James H. Kryklywy d,e, Ewan A. Macpherson c,f, Steven G. Greening b,e, Derek G.V. Mitchell a,b,d,e,⁎ a Department of Psychiatry, University of Western Ontario, London, Ontario N6A 5A5, Canada b Department of Anatomy & Cell Biology, University of Western Ontario, London, Ontario N6A 5A5, Canada c National Centre for Audiology, University of Western Ontario, London, Ontario N6A 5A5, Canada d Graduate Program in Neuroscience, University of Western Ontario, London, Ontario N6A 5A5, Canada e Brain and Mind Institute, University of Western Ontario, London, Ontario N6A 5A5, Canada f School of Communication Sciences and Disorders, University of Western Ontario, London, Ontario N6A 5A5, Canada article info abstract Article history: Auditory cortices can be separated into dissociable processing pathways similar to those observed in the vi- Accepted 8 May 2013 sual domain. Emotional stimuli elicit enhanced neural activation within sensory cortices when compared to Available online 24 May 2013 neutral stimuli. This effect is particularly notable in the ventral visual stream. Little is known, however, about how emotion interacts with dorsal processing streams, and essentially nothing is known about the impact of Keywords: emotion on auditory stimulus localization. In the current study, we used fMRI in concert with individualized Auditory localization Emotion auditory virtual environments to investigate the effect of emotion during an auditory stimulus localization fi Auditory processing pathways task. Surprisingly, participants were signi cantly slower to localize emotional relative to neutral sounds. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -
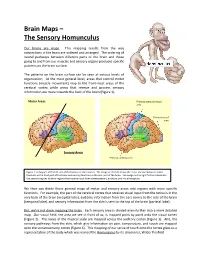
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Subdivisions of Auditory Cortex and Processing Streams in Primates
Colloquium Subdivisions of auditory cortex and processing streams in primates Jon H. Kaas*† and Troy A. Hackett‡ Departments of †Psychology and ‡Hearing and Speech Sciences, Vanderbilt University, Nashville, TN 37240 The auditory system of monkeys includes a large number of histochemical studies in chimpanzees and humans, and nonin- interconnected subcortical nuclei and cortical areas. At subcortical vasive functional studies in humans. levels, the structural components of the auditory system of mon- keys resemble those of nonprimates, but the organization at The Core Areas of Auditory Cortex cortical levels is different. In monkeys, the ventral nucleus of the Originally, auditory cortex of monkeys was thought to be orga- medial geniculate complex projects in parallel to a core of three nized much as in cats, with a single primary area, AI, in the primary-like auditory areas, AI, R, and RT, constituting the first cortex of the lower bank of the lateral sulcus and a second area, stage of cortical processing. These areas interconnect and project AII, deeper in the sulcus (e.g., ref. 3). This concept fits well with to the homotopic and other locations in the opposite cerebral the early view that auditory, somatosensory, and visual systems hemisphere and to a surrounding array of eight proposed belt all have two fields. However, we now know that primates have areas as a second stage of cortical processing. The belt areas in turn a number of sensory representations for each modality, and project in overlapping patterns to a lateral parabelt region with at several somatosensory and auditory fields can be considered least rostral and caudal subdivisions as a third stage of cortical primary or primary like in character. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

Acetylcholinesterase Staining in Human Auditory and Language
Acetylcholinesterase Staining in Human Jeffrey J. Hutsler and Michael S. Gazzaniga Auditory and Language Cortices: Center for Neuroscience, University of California, Davis Regional Variation of Structural Features California 95616 Cholinergic innovation of the cerebral neocortex arises from the basal 1974; Greenfield, 1984, 1991; Robertson, 1987; Taylor et al., forebrain and projects to all cortical regions. Acetylcholinesterase 1987; Krisst, 1989; Small, 1989, 1990). AChE-containing axons (AChE), the enzyme responsible for deactivating acetylcholine, is found of the cerebral cortex are also immunoreactive for choline within both cholinergic axons arising from the basal forebrain and a acetyltransferase (ChAT) and are therefore known to be cho- Downloaded from subgroup of pyramidal cells in layers III and V of the cerebral cortex. linergic (Mesulam and Geula, 1992). This pattern of staining varies with cortical location and may contrib- AChE-containing pyramidal cells of layers III and V are not ute uniquely to cortical microcircuhry within functionally distinct cholinergic (Mesulam and Geula, 1991), but it has been sug- regions. To explore this issue further, we examined the pattern of AChE gested that they are cholinoceptive (Krnjevic and Silver, 1965; staining within auditory, auditory association, and putative language Levey et al., 1984; Mesulam et al., 1984b). In support of this regions of whole, postmortem human brains. notion, layer in and V pyramidal cell excitability can be mod- http://cercor.oxfordjournals.org/ The density and distribution of acetylcholine-containing axons and ulated by the application of acetylcholine in the slice prepa- pyramidal cells vary systematically as a function of auditory process- ration (McCormick and Williamson, 1989). Additionally, mus- ing level. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -
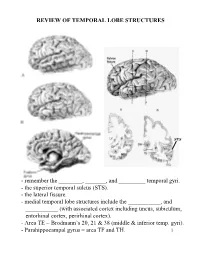
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -

The Depth Asymmetry of Superior Temporal Sulcus
New human-specific brain landmark: The depth asymmetry of superior temporal sulcus François Leroya,1, Qing Caib, Stephanie L. Bogartc, Jessica Duboisa, Olivier Coulond, Karla Monzalvoa, Clara Fischere, Hervé Glasela, Lise Van der Haegenf, Audrey Bénézita, Ching-Po Ling, David N. Kennedyh, Aya S. Iharai, Lucie Hertz-Pannierj, Marie-Laure Moutardk, Cyril Pouponl, Marc Brysbaerte, Neil Robertsm, William D. Hopkinsc, Jean-François Mangine, and Ghislaine Dehaene-Lambertza aCognitive Neuroimaging Unit, U992, eAnalysis and Processing of Information Unit, jClinical and Translational Applications Research Unit, U663, and lNuclear Magnetic Resonance Imaging and Spectroscopy Unit, Office of Atomic Energy and Alternative Energies (CEA), INSERM, NeuroSpin, Gif-sur-Yvette 91191, France; bShanghai Key Laboratory of Brain Functional Genomics, East China Normal University, Shanghai 200241, China; cDivision of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA 30322; dLaboratory for Systems and Information Science, UMR CNRS 7296, Aix-Marseille University, Marseille 13284, France; fDepartment of Experimental Psychology, Ghent University, Ghent B-9000, Belgium; gInstitute of Neuroscience, National Yang-Ming University, Taipei City 112, Taiwan; hCenter for Morphometric Analysis, Neuroscience Center, Massachusetts General Hospital, Boston, MA 02114; iCenter for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka 565-0871 Japan; kNeuropediatrics Department, Trousseau