Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

'What' but Not 'Where' Auditory Processing Pathway
NeuroImage 82 (2013) 295–305 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Emotion modulates activity in the ‘what’ but not ‘where’ auditory processing pathway James H. Kryklywy d,e, Ewan A. Macpherson c,f, Steven G. Greening b,e, Derek G.V. Mitchell a,b,d,e,⁎ a Department of Psychiatry, University of Western Ontario, London, Ontario N6A 5A5, Canada b Department of Anatomy & Cell Biology, University of Western Ontario, London, Ontario N6A 5A5, Canada c National Centre for Audiology, University of Western Ontario, London, Ontario N6A 5A5, Canada d Graduate Program in Neuroscience, University of Western Ontario, London, Ontario N6A 5A5, Canada e Brain and Mind Institute, University of Western Ontario, London, Ontario N6A 5A5, Canada f School of Communication Sciences and Disorders, University of Western Ontario, London, Ontario N6A 5A5, Canada article info abstract Article history: Auditory cortices can be separated into dissociable processing pathways similar to those observed in the vi- Accepted 8 May 2013 sual domain. Emotional stimuli elicit enhanced neural activation within sensory cortices when compared to Available online 24 May 2013 neutral stimuli. This effect is particularly notable in the ventral visual stream. Little is known, however, about how emotion interacts with dorsal processing streams, and essentially nothing is known about the impact of Keywords: emotion on auditory stimulus localization. In the current study, we used fMRI in concert with individualized Auditory localization Emotion auditory virtual environments to investigate the effect of emotion during an auditory stimulus localization fi Auditory processing pathways task. Surprisingly, participants were signi cantly slower to localize emotional relative to neutral sounds. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Interoception: the Sense of the Physiological Condition of the Body AD (Bud) Craig
500 Interoception: the sense of the physiological condition of the body AD (Bud) Craig Converging evidence indicates that primates have a distinct the body share. Recent findings that compel a conceptual cortical image of homeostatic afferent activity that reflects all shift resolve these issues by showing that all feelings from aspects of the physiological condition of all tissues of the body. the body are represented in a phylogenetically new system This interoceptive system, associated with autonomic motor in primates. This system has evolved from the afferent control, is distinct from the exteroceptive system (cutaneous limb of the evolutionarily ancient, hierarchical homeostatic mechanoreception and proprioception) that guides somatic system that maintains the integrity of the body. These motor activity. The primary interoceptive representation in the feelings represent a sense of the physiological condition of dorsal posterior insula engenders distinct highly resolved the entire body, redefining the category ‘interoception’. feelings from the body that include pain, temperature, itch, The present article summarizes this new view; more sensual touch, muscular and visceral sensations, vasomotor detailed reviews are available elsewhere [1,2]. activity, hunger, thirst, and ‘air hunger’. In humans, a meta- representation of the primary interoceptive activity is A homeostatic afferent pathway engendered in the right anterior insula, which seems to provide Anatomical characteristics the basis for the subjective image of the material self as a feeling Cannon [3] recognized that the neural processes (auto- (sentient) entity, that is, emotional awareness. nomic, neuroendocrine and behavioral) that maintain opti- mal physiological balance in the body, or homeostasis, must Addresses receive afferent inputs that report the condition of the Atkinson Pain Research Laboratory, Division of Neurosurgery, tissues of the body. -

The Contribution of Sensory System Functional Connectivity Reduction to Clinical Pain in Fibromyalgia
1 The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia Jesus Pujol1,2, Dídac Macià1, Alba Garcia-Fontanals3, Laura Blanco-Hinojo1,4, Marina López-Solà1,5, Susana Garcia-Blanco6, Violant Poca-Dias6, Ben J Harrison7, Oren Contreras-Rodríguez1, Jordi Monfort8, Ferran Garcia-Fructuoso6, Joan Deus1,3 1MRI Research Unit, CRC Mar, Hospital del Mar, Barcelona, Spain. 2Centro Investigación Biomédica en Red de Salud Mental, CIBERSAM G21, Barcelona, Spain. 3Department of Clinical and Health Psychology, Autonomous University of Barcelona, Spain. 4Human Pharmacology and Neurosciences, Institute of Neuropsychiatry and Addiction, Hospital del Mar Research Institute, Barcelona, Spain. 5Department of Psychology and Neuroscience. University of Colorado, Boulder, Colorado. 6Rheumatology Department, Hospital CIMA Sanitas, Barcelona, Spain. 7Melbourne Neuropsychiatry Centre, Department of Psychiatry, The University of Melbourne, Melbourne, Australia. 8Rheumatology Department, Hospital del Mar, Barcelona, Spain The submission contains: The main text file (double-spaced 39 pages) Six Figures One supplemental information file Corresponding author: Dr. Jesus Pujol Department of Magnetic Resonance, CRC-Mar, Hospital del Mar Passeig Marítim 25-29. 08003, Barcelona, Spain Email: [email protected] Telephone: + 34 93 221 21 80 Fax: + 34 93 221 21 81 Synopsis/ Summary Clinical pain in fibromyalgia is associated with functional changes at different brain levels in a pattern suggesting a general weakening of sensory integration. 2 Abstract Fibromyalgia typically presents with spontaneous body pain with no apparent cause and is considered pathophysiologically to be a functional disorder of somatosensory processing. We have investigated potential associations between the degree of self-reported clinical pain and resting-state brain functional connectivity at different levels of putative somatosensory integration. -
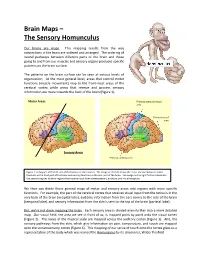
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Subdivisions of Auditory Cortex and Processing Streams in Primates
Colloquium Subdivisions of auditory cortex and processing streams in primates Jon H. Kaas*† and Troy A. Hackett‡ Departments of †Psychology and ‡Hearing and Speech Sciences, Vanderbilt University, Nashville, TN 37240 The auditory system of monkeys includes a large number of histochemical studies in chimpanzees and humans, and nonin- interconnected subcortical nuclei and cortical areas. At subcortical vasive functional studies in humans. levels, the structural components of the auditory system of mon- keys resemble those of nonprimates, but the organization at The Core Areas of Auditory Cortex cortical levels is different. In monkeys, the ventral nucleus of the Originally, auditory cortex of monkeys was thought to be orga- medial geniculate complex projects in parallel to a core of three nized much as in cats, with a single primary area, AI, in the primary-like auditory areas, AI, R, and RT, constituting the first cortex of the lower bank of the lateral sulcus and a second area, stage of cortical processing. These areas interconnect and project AII, deeper in the sulcus (e.g., ref. 3). This concept fits well with to the homotopic and other locations in the opposite cerebral the early view that auditory, somatosensory, and visual systems hemisphere and to a surrounding array of eight proposed belt all have two fields. However, we now know that primates have areas as a second stage of cortical processing. The belt areas in turn a number of sensory representations for each modality, and project in overlapping patterns to a lateral parabelt region with at several somatosensory and auditory fields can be considered least rostral and caudal subdivisions as a third stage of cortical primary or primary like in character. -

Neural Mapping of Direction and Frequency in the Cricket Cercal Sensory System
The Journal of Neuroscience, March 1, 1999, 19(5):1771–1781 Neural Mapping of Direction and Frequency in the Cricket Cercal Sensory System Sussan Paydar,2 Caitlin A. Doan,2 and Gwen A. Jacobs1 1Center for Computational Biology, Montana State University, Bozeman, Montana 59717, and 2Department of Molecular and Cell Biology, University of California, Berkeley, California 94720 Primary mechanosensory receptors and interneurons in the segregation was investigated, by calculating the relative over- cricket cercal sensory system are sensitive to the direction and lap between the long and medium-length hair afferents with the frequency of air current stimuli. Receptors innervating long dendrites of two interneurons that are known to have different mechanoreceptor hairs (.1000 mm) are most sensitive to low- frequency sensitivities. Both interneurons were shown to have frequency air currents (,150 Hz); receptors innervating nearly equal anatomical overlap with long and medium hair medium-length hairs (900–500 mm) are most sensitive to higher afferents. Thus, the differential overlap of these interneurons frequency ranges (150–400 Hz). Previous studies demonstrated with the two different classes of afferents was not adequate to that the projection pattern of the synaptic arborizations of long explain the observed frequency selectivity of the interneurons. hair receptor afferents form a continuous map of air current Other mechanisms such as selective connectivity between direction within the terminal abdominal ganglion (Jacobs and subsets of afferents and interneurons and/or differences in Theunissen, 1996). We demonstrate here that the projection interneuron biophysical properties must play a role in establish- pattern of the medium-length hair afferents also forms a con- ing the frequency selectivities of these interneurons. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

Portia Perceptions: the Umwelt of an Araneophagic Jumping Spider
Portia Perceptions: The Umwelt of an Araneophagic Jumping 1 Spider Duane P. Harland and Robert R. Jackson The Personality of Portia Spiders are traditionally portrayed as simple, instinct-driven animals (Savory, 1928; Drees, 1952; Bristowe, 1958). Small brain size is perhaps the most compelling reason for expecting so little flexibility from our eight-legged neighbors. Fitting comfortably on the head of a pin, a spider brain seems to vanish into insignificance. Common sense tells us that compared with large-brained mammals, spiders have so little to work with that they must be restricted to a circumscribed set of rigid behaviors, flexibility being a luxury afforded only to those with much larger central nervous systems. In this chapter we review recent findings on an unusual group of spiders that seem to be arachnid enigmas. In a number of ways the behavior of the araneophagic jumping spiders is more comparable to that of birds and mammals than conventional wisdom would lead us to expect of an arthropod. The term araneophagic refers to these spiders’ preference for other spiders as prey, and jumping spider is the common English name for members of the family Saltici- dae. Although both their common and the scientific Latin names acknowledge their jumping behavior, it is really their unique, complex eyes that set this family of spiders apart from all others. Among spiders (many of which have very poor vision), salticids have eyes that are by far the most specialized for resolving fine spatial detail. We focus here on the most extensively studied genus, Portia. Before we discuss the interrelationship between the salticids’ uniquely acute vision, their predatory strategies, and their apparent cognitive abilities, we need to offer some sense of what kind of animal a jumping spider is; to do this, we attempt to offer some insight into what we might call Portia’s personality. -
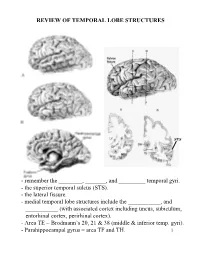
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________.