Supplemental Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10041.Full.Pdf
The Journal of Neuroscience, July 23, 2014 • 34(30):10041–10054 • 10041 Systems/Circuits Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders Sarah R. Heilbronner and Suzanne N. Haber Department of Pharmacology and Physiology, University of Rochester Medical Center, Rochester, New York 14642 The cingulum bundle (CB) is one of the brain’s major white matter pathways, linking regions associated with executive function, decision-making, and emotion. Neuroimaging has revealed that abnormalities in particular locations within the CB are associated with specific psychiatric disorders, including depression and bipolar disorder. However, the fibers using each portion of the CB remain unknown. In this study, we used anatomical tract-tracing in nonhuman primates (Macaca nemestrina, Macaca fascicularis, Macaca mulatta)toexaminetheorganizationofspecificcingulate,noncingulatefrontal,andsubcorticalpathwaysthroughtheCB.Thegoalswere as follows: (1) to determine connections that use the CB, (2) to establish through which parts of the CB these fibers travel, and (3) to relate the CB fiber pathways to the portions of the CB identified in humans as neurosurgical targets for amelioration of psychiatric disorders. Results indicate that cingulate, noncingulate frontal, and subcortical fibers all travel through the CB to reach both cingulate and noncin- gulate targets. However, many brain regions send projections through only part, not all, of the CB. For example, amygdala fibers are not present in the caudal portion of the dorsal CB. These results allow segmentation of the CB into four unique zones. We identify the specific connections that are abnormal in psychiatric disorders and affected by neurosurgical interventions, such as deep brain stimulation and cingulotomy. -
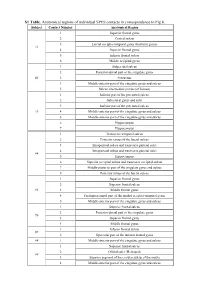
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Correction for Partial-Volume Effects on Brain Perfusion SPECT in Healthy Men
Correction for Partial-Volume Effects on Brain Perfusion SPECT in Healthy Men Hiroshi Matsuda, MD1; Takashi Ohnishi, MD1; Takashi Asada, MD2; Zhi-jie Li, MD1,3; Hidekazu Kanetaka, MD1; Etsuko Imabayashi, MD1; Fumiko Tanaka, MD1; and Seigo Nakano, MD4 1Department of Radiology, National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry, Tokyo, Japan; 2Department of Neuropsychiatry, Institute of Clinical Medicine, University of Tsukuba, Ibaraki, Japan; 3Department of Nuclear Medicine, The Second Clinical Hospital of China Medical University, Shen-Yang City, China; and 4Department of Geriatric Medicine, National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry, Tokyo, Japan The limited spatial resolution of SPECT scanners does not allow Functional changes in the brains of healthy elderly people an exact measurement of the local radiotracer concentration in and patients with neurodegenerative disorders have been brain tissue because partial-volume effects (PVEs) underesti- studied widely by SPECT. However, due to the limited mate concentration in small structures of the brain. The aim of this study was to determine which brain structures show greater spatial resolution of SPECT, the accurate measurement of influence of PVEs in SPECT studies on healthy volunteers and to tracer concentration in brain structures depends on several investigate aging effects on SPECT after the PVE correction. physical limitations—particularly, the relation between ob- Methods: Brain perfusion SPECT using 99mTc-ethylcysteinate ject size and scanner spatial resolution. This relation, known dimer was performed in 52 healthy men, 18–86 y old. The as the partial-volume effect (PVE), biases the measured regional cerebral blood flow (rCBF) was noninvasively measured concentration in small structures by diminishing the true using graphical analysis. -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Neural Populations in Human Posteromedial Cortex Display Opposing Responses During Memory and Numerical Processing
Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing Brett L. Foster, Mohammad Dastjerdi, and Josef Parvizi1 Laboratory of Behavioral and Cognitive Neurology, Department of Neurology and Neurological Sciences, Stanford Human Intracranial Cognitive Electrophysiology Program, Stanford University School of Medicine, Stanford University, Stanford, CA 94305 Edited by Marcus E. Raichle, Washington University in St. Louis, St. Louis, MO, and approved August 7, 2012 (received for review April 23, 2012) Our understanding of the human default mode network derives rate is correlated with behavioral performance, where lower levels primarily from neuroimaging data but its electrophysiological of PCC firing suppression are associated with longer reaction correlates remain largely unexplored. To address this limitation, times (RTs) and higher error rate, a correlation also previously we recorded intracranially from the human posteromedial cortex reported with fMRI in humans (11, 12). Similarly, direct cortical (PMC), a core structure of the default mode network, during various recordings using electrocorticography (ECoG) from human conditions of internally directed (e.g., autobiographical memory) as DMN (13–16) have shown suppression of broadband γ-power [a opposed to externally directed focus (e.g., arithmetic calculation). correlate of population firing rate (17, 18)] during attentional We observed late-onset (>400 ms) increases in broad high γ-power effort. More recently, this broadband suppression across human (70–180 Hz) within PMC subregions during memory retrieval. High DMN regions has also been shown to correlate with behavioral γ-power was significantly reduced or absent when subjects re- performance (16). Taken together, these findings have provided trieved self-referential semantic memories or responded to self- clear electrophysiological evidence for DMN suppression. -

The Pre/Parasubiculum: a Hippocampal Hub for Scene- Based Cognition? Marshall a Dalton and Eleanor a Maguire
Available online at www.sciencedirect.com ScienceDirect The pre/parasubiculum: a hippocampal hub for scene- based cognition? Marshall A Dalton and Eleanor A Maguire Internal representations of the world in the form of spatially which posits that one function of the hippocampus is to coherent scenes have been linked with cognitive functions construct internal representations of scenes in the ser- including episodic memory, navigation and imagining the vice of memory, navigation, imagination, decision-mak- future. In human neuroimaging studies, a specific hippocampal ing and a host of other functions [11 ]. Recent inves- subregion, the pre/parasubiculum, is consistently engaged tigations have further refined our understanding of during scene-based cognition. Here we review recent evidence hippocampal involvement in scene-based cognition. to consider why this might be the case. We note that the pre/ Specifically, a portion of the anterior medial hippocam- parasubiculum is a primary target of the parieto-medial pus is consistently engaged by tasks involving scenes temporal processing pathway, it receives integrated [11 ], although it is not yet clear why a specific subre- information from foveal and peripheral visual inputs and it is gion of the hippocampus would be preferentially contiguous with the retrosplenial cortex. We discuss why these recruited in this manner. factors might indicate that the pre/parasubiculum has privileged access to holistic representations of the environment Here we review the extant evidence, drawing largely from and could be neuroanatomically determined to preferentially advances in the understanding of visuospatial processing process scenes. pathways. We propose that the anterior medial portion of the hippocampus represents an important hub of an Address extended network that underlies scene-related cognition, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, and we generate specific hypotheses concerning the University College London, 12 Queen Square, London WC1N 3BG, UK functional contributions of hippocampal subfields. -

Translingual Neural Stimulation with the Portable Neuromodulation
Translingual Neural Stimulation With the Portable Neuromodulation Stimulator (PoNS®) Induces Structural Changes Leading to Functional Recovery In Patients With Mild-To-Moderate Traumatic Brain Injury Authors: Jiancheng Hou,1 Arman Kulkarni,2 Neelima Tellapragada,1 Veena Nair,1 Yuri Danilov,3 Kurt Kaczmarek,3 Beth Meyerand,2 Mitchell Tyler,2,3 *Vivek Prabhakaran1 1. Department of Radiology, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin, USA 2. Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, Wisconsin, USA 3. Department of Kinesiology, University of Wisconsin-Madison, Madison, Wisconsin, USA *Correspondence to [email protected] Disclosure: Dr Tyler, Dr Danilov, and Dr Kaczmarek are co-founders of Advanced Neurorehabilitation, LLC, which holds the intellectual property rights to the PoNS® technology. Dr Tyler is a board member of NeuroHabilitation Corporation, a wholly- owned subsidiary of Helius Medical Technologies, and owns stock in the corporation. The other authors have declared no conflicts of interest. Acknowledgements: Professional medical writing and editorial assistance were provided by Kelly M. Fahrbach, Ashfield Healthcare Communications, part of UDG Healthcare plc, funded by Helius Medical Technologies. Dr Tyler, Dr Kaczmarek, Dr Danilov, Dr Hou, and Dr Prabhakaran were being supported by NHC-TBI-PoNS-RT001. Dr Hou, Dr Kulkarni, Dr Nair, Dr Tellapragada, and Dr Prabhakaran were being supported by R01AI138647. Dr Hou and Dr Prabhakaran were being supported by P01AI132132, R01NS105646. Dr Kulkarni was being supported by the Clinical & Translational Science Award programme of the National Center for Research Resources, NCATS grant 1UL1RR025011. Dr Meyerand, Dr Prabhakaran, Dr Nair was being supported by U01NS093650. -

Study of Extra-Visual Resting-State Networks in Pituitary Adenoma Patients with Vision Restoration
Study of Extra-visual Resting-state Networks in Pituitary Adenoma Patients With Vision Restoration Fuyu Wang ( [email protected] ) Department of Neurosurgery, Chinese PLA General Hospital, Beijing, China https://orcid.org/0000- 0003-2232-5423 Peng Wang PLAGH: Chinese PLA General Hospital Ze Li PLAGH: Chinese PLA General Hospital Tao Zhou Chinese PLA General Hospital Xianghui Meng Chinese PLA General Hospital Jinli Jiang Chinese PLA General Hospital Research article Keywords: cross-modal plasticity, default mode network, resting-state functional magnetic resonance imaging, salience network, visual improvement Posted Date: December 29th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-133978/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/23 Abstract Background: Pituitary adenoma(PA) may compress the optic apparatus and cause impaired vision. Some patients can get improved vision rapidly after surgery. During the early time after surgery, however, the change of neurofunction in extra-visual cortex and higher cognitive cortex is still yet to be explored so far. Objective: Our study is focused on the changes in the extra-visual resting-state networks in PA patients after vision restoration. Methods:We recruited 14 PA patients with visual improvement after surgery. The functional connectivity (FC) of 6 seeds (auditory cortex (A1), Broca's area, posterior cingulate cortex (PCC)for default mode network (DMN), right caudal anterior cingulate cortex for salience network(SN) and left dorsolateral prefrontal cortex for excecutive control network (ECN)) were evaluated. A paired t-test was conducted to identify the differences between two groups. -

Morphology and Digitally Aided Morphometry of the Human Paracentral Lobule
Folia Morphol. Vol. 72, No. 1, pp. 10–16 DOI: 10.5603/FM.2013.0002 O R I G I N A L A R T I C L E Copyright © 2013 Via Medica ISSN 0015–5659 www.fm.viamedica.pl Morphology and digitally aided morphometry of the human paracentral lobule G. Spasojević1, S. Malobabić2, O. Pilipović-Spasojević3, N. Djukić Macut4, A. Maliković2 1Department of Anatomy, Faculty of Medicine, University of Banja Luka, Banja Luka, Republic of Srpska, Bosnia and Herzegovina 2Institute of Anatomy “Dr Niko Miljanić”, Faculty of Medicine, University of Belgrade, Belgrade, Serbia 3Department of Physical Medicine and Rehabilitation “Dr Miroslav Zotović”, Banja Luka, Republic of Srpska, Bosnia and Herzegovina 4Department of Anatomy, Faculty of Medicine, University of Kosovska Mitrovica (Priština), Serbia [Received 13 October 2012; Accepted 17 December 2012] The human paracentral lobule, the junction of the precentral and postcentral gyri at the medial hemispheric surface, contains several important functional regions, and its variable morphology requires exact morphological and quantita- tive data. In order to obtain precise data we investigated the morphology of the paracentral lobule and quantified its visible (extrasulcal) surface. This surface cor- responds to commonly used magnetic resonance imaging scout images. We stud- ied 84 hemispheres of adult persons (42 brains; 26 males and 16 females; 20– –65 years) fixed in neutral formalin for at least 4 weeks. The medial hemispheric surface was photographed at standard distance and each digital photo was ca- librated. Using the intercommissural line system (commissura anterior-commis- sura posterior or CA-CP line), we performed standardised measurements of the paracentral lobule. -

Supporting Information for “Endocast Morphology of Homo Naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, S
Supporting Information for “Endocast Morphology of Homo naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, Shawn D. Hurst, Heather M. Garvin, P. Thomas Schoenemann, William B. Vanti, Lee R. Berger, and John Hawks What follows are our descriptions, illustrations, some basic interpretation, and a more specific discussion of the functional, comparative, and taxonomic issues surrounding these hominins. We use the neuroanatomical nomenclature from Duvernoy (19). DH1 Occipital The DH1 occipital fragment (Figs 1, S2) measures ca 105 mm in width between left temporo- occipital incisure and right sigmoid sinus. It is 61 mm in height on the left side, and 47 mm on the right side. The fragment covers the entire left and mostly complete right occipital lobes. The lobes are strongly asymmetrical, with the left clearly larger than the right, and more posteriorly protruding. There are faint traces of a lateral remnant of the lunate sulcus on the left side, and a dorsal bounding lunate as well (#4 and #6 in Fig 1). The right side shows a very small groove at the end of the lateral sinus, which could be a remnant of the lunate sulcus. The major flow from the longitudinal sinus is to the right. Small portions of both cerebellar lobes, roughly 15 mm in height are present. There is a suggestion of a great cerebellar sulcus on the right side. The width from the left lateral lunate impression to the midline is 43mm. The distance from left occipital pole (the most posteriorly projecting point, based on our best estimate of the proper orientation) to the mid-sagittal plane is 30 mm. -

On the Scent of Human Olfactory Orbitofrontal Cortex: Meta-Analysis and Comparison to Non-Human Primates
Brain Research Reviews 50 (2005) 287 – 304 www.elsevier.com/locate/brainresrev Review On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates Jay A. Gottfrieda,*, David H. Zaldb aDepartment of Neurology and the Cognitive Neurology and Alzheimer’s Disease Center, Northwestern University Feinberg School of Medicine, 320 E. Superior St., Searle 11-453, Chicago, IL 60611, USA bDepartment of Psychology, Vanderbilt University, Nashville, TN 37240, USA Accepted 25 August 2005 Available online 6 October 2005 Abstract It is widely accepted that the orbitofrontal cortex (OFC) represents the main neocortical target of primary olfactory cortex. In non-human primates, the olfactory neocortex is situated along the basal surface of the caudal frontal lobes, encompassing agranular and dysgranular OFC medially and agranular insula laterally, where this latter structure wraps onto the posterior orbital surface. Direct afferent inputs arrive from most primary olfactory areas, including piriform cortex, amygdala, and entorhinal cortex, in the absence of an obligatory thalamic relay. While such findings are almost exclusively derived from animal data, recent cytoarchitectonic studies indicate a close anatomical correspondence between non-human primate and human OFC. Given this cross-species conservation of structure, it has generally been presumed that the olfactory projection area in human OFC occupies the same posterior portions of OFC as seen in non-human primates. This review questions this assumption by providing a critical survey of the localization of primate and human olfactory neocortex. Based on a meta-analysis of human functional neuroimaging studies, the region of human OFC showing the greatest olfactory responsivity appears substantially rostral and in a different cytoarchitectural area than the orbital olfactory regions as defined in the monkey.