Axis Scientific Deluxe Brain A-106198
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

A Cyclops and a Synotus by K
J Neurol Psychopathol: first published as 10.1136/jnnp.s1-17.65.48 on 1 July 1936. Downloaded from 48 ORIGINAL PAPERS A CYCLOPS AND A SYNOTUS BY K. H. BOUMAN, AMSTERDAM, AND V. W. D. SCHENK, TiH HAGUE INTRODUCTION ONLY a small number of cases of cyclopia in human beings and mammals have been minutely examined. The number becomes still smaller if a more or less complete microscopic investigation of the central nervous system is stipulated. It is really only the cases of Davidson Black and Winkler and perhaps that of Naegli which answer this requirement. In contrast therewith there is an abundance of experimental studies in this field in urodela and other lower classes of animals. For all that, unanimity does not by any means prevail here, although the Protected by copyright. views of Stockard and his followers-who held that the first determination of the eye lay unpaired in the median line-and those of Spemann-who pointed to a paired rudiment from the outset, which views were originally diametrically opposed-appear to have drawn somewhat nearer to each other in recent years. Woerdeman, for instance, found that the paired rudiment of the eye shifts its position laterally downwards very early (when the folds of the medullary plate become visible) and he rightly says that this is not the same as Stockard's lateral growth of an unpaired eye rudiment. Yet, by saying this, he admits certain changes and growth conditions to which Fischel, for instance, did not do full justice. E. Manchot, on the other hand, who defends Stockard's views, admits that between the two regions of the eye http://jnnp.bmj.com/ rudiment there must be a tract of brain tissue (lamina terminalis and regio chiasmatica). -
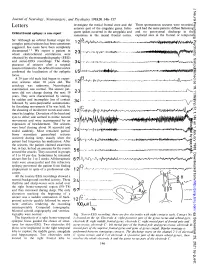
Letters Anterior Part of the Cingulate Gyrus
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.1.146 on 1 January 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:146-157 investigate the mesial frontal zone and the Three spontaneous seizures were recorded, Letters anterior part of the cingulate gyrus. Infre- each had the same pattern: diffuse flattening quent spikes occurred in the amygdala and and no in the Orbital frontal epilepsy: a case report paroxysmal discharge sometimes in the mesial frontal cortex. explored sites in the frontal or temporal Sir: Although an orbital frontal origin for A complex partial seizures has been sometimes 12 suggested, few cases have been completely documented.1 2 We report a patient in 23 whom electroclinical correlations were obtained by electroencephalography (EEG) and stereo-EEG recordings. The disap- 3.4 I-w VI,-#a+r - pearance of seizures after a surgical resection limited to the orbital frontal cortex confirmed the localisation of the epileptic 4.5 o I\A-.ViJV "fJ4 WOO focus. A 29 year old male had begun to experi- B ence seizures when 10 years old. The aetiology was unknown. Neurological examination was normal. The seizure pat- terns did not change during the next 19 23 ., years. They were characterised by staring, r by sudden and incomplete loss of contact -- - -. , A- followed by semi-purposeful automatisms, 3-4 v by thrashing movements if he was held, by the shouting of incoherent words and some- times by laughter. Deviation of the head and eyes to either side seemed to mimic natural Protected by copyright. -
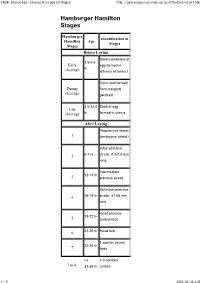
Hamburger Hamilton Stages
UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm Hamburger Hamilton Stages Hamburger Identification of Hamilton Age Stages Stages Before Laying Shell membrane of 3.5-4.5 Early egg formed in hr cleavage isthmus of oviduct Germ wall formed During from marginal cleavage periblast 4.5-24.0 Shell of egg Late cleavage hr formed in uterus After Laying Preprimitive streak 1 (embryonic shield) Initial primitive 2 6-7 hr streak, 0.3-0.5 mm long Intermediate 12-13 hr 3 primitive streak Definitive primitive 4 18-19 hr streak, ±1.88 mm long Head process 19-22 hr 5 (notochord) 6 23-25 hr Head fold 1 somite; neural 23-26 hr 7 folds ca. 1-3 somites; 7 to 8- 23-26 hr coelom 1 / 5 2007/03/20 9:05 UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm 4 somites; blood 26-29 hr 8 islands 7 somites; primary 29-33 hr 9 optic vesicles 8-9 somites; 9+ to 10- ca. 33 hr anterior amniotic fold 10 somites; 3 10 33-38 hr primary brain vesicles 13 somites; 5 11 40-45 hr neuromeres of hindbrain 16 somites; 45-49 hr 12 telencephalon 19 somites; 13 48-52 hr atrioventricular canal ca. 20-21 somites; tail 13+ to 14- 50-52 hr bud 22 somites; trunk flexure; visceral 50-53 hr 14 arches I and II, clefts 1 and 2 23 somites; ca. premandibular 14+ to 15- 50-54 hr head cavities 24-27 somites; 15 50-55 hr visceral arch III, cleft 3 26-28 somites; 16 51-56 hr wing bud; posterior 2 / 5 2007/03/20 9:05 UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm -

Nervous System Cns
THE NERVOUS SYSTEM CNS • Function The Spinal Cord • General Structure • Enclosed In: • Neural Foramen – length • Connects With: • Foramen magnum – need for protection Coverings • Coverings: • meninges (3) • Subarachnoid space • location • composition • diagnostic use Spinal Nerves • Caudal equina Finer Structures of Spinal Cord • Gray Matter • composition • function • horns (2) • horns form roots (2) • gray commisure • central canal Finer Structures of Spinal Cord • White matter • arrangement • columns contain tracts • description • names The Brain •General Structure •Protection •Skeletal •Membranous The Brain • Development • Neural Plate • Neural Tube and 3 swellings The Brain • Other Structures • Ventricles • Foramen of Monroe • Cerebral Aqueduct The Brain - finer structures • Brain Stem • Medulla • location • connection • gray matter vs. white matter • function • kinds of reflexes The Brain • Brain stem • Pons • Structure • Composition • Nerves The Brain • Brain stem • Midbrain • Location • Composition: • Cerebral peduncles • Substantia nigra • Tegmentum • Corpora quadrigemina • Cerebral aqueduct The Brain • Cerebellum • Location • Structure • Cortex The Brain • Cerebellum • White Matter • Cerebellar Nuclei • Dentate Nuclei • Furrows • Divisions • Functions The Brain • Interbrain • Contains structures (2) • Location • How functions were determined Interbrain • The Thalamus • Function • Result of Injury Interbrain • The Hypothalmus • Function • Reason for these functions • Result of Injury The Brain • The Cerebrum • Size • Complexity • -

Metabolic Responses in Discrete, Mice Brain Regions Like CC, CG, H and CQ During PTZ Induced Epileptic Seizures
Current Neurobiology 2012; 3 (1): 31-38 ISSN 0975-9042 Scientific Publishers of India Metabolic responses in discrete, Mice brain regions like CC, CG, H and CQ during PTZ induced epileptic seizures. K. K. Therisa and P.V. Desai Department of Zoology, Goa University, Taleigao Plateau, Goa 403206, India. Abstract Epilepsy, a neurological disorder with recurrent seizures, involves disruption of different metabolic enzymes and its related metabolites altering the normal processes of metabolism, either during the onset or post epilepsy in the brain. In the present work, it is convincingly, observed that the Mice brain regions such as Corpus callosum (CC), Cingulate gyrus (CG), Hippocampus (H) and Corpora quadrigemina (CQ) shows significant changes in the activi- ties of metabolic enzymes such as AST, ALT, LDH; ATPases like Na +, K +-ATPase, Mg 2+ - ATPase, Ca 2+ -ATPase along with their metabolites such as Glucose, Pyruvate, Lactate and Glutamate, altering the metabolic integrity during Pentylenetetrazole (PTZ) induced epilep- tic seizure. We report from the present work that, H and CG are affected completely during epileptic seizure as compared to its control but CC and CQ shows partly altered as far as metabolism in brain is concerned. Keywords: Corpus callosum, Hippocampus, Cingulate gyrus, Corpora Quadrigemina, Metabolic enzymes and metabo- lites. Accepted April 07 2012 Introduction may overlap with other frontal lobe epilepsy syndromes. But, an aberrant behaviors observed in epileptic patients Epilepsy, involves a disruption of brain energy homeosta- completely resolved after lesionectomy of Cingulate sis and is potentially manageable through principles of gyrus [6]. Hippocampus and Cingulate gyrus forms the metabolic control theory. -

Occipital Sulci Patterns in Patients with Schizophrenia and Migraine Headache Using Magnetic Resonance Imaging (MRI)
ORIGINAL ARTICLE Occipital sulci patterns in patients with schizophrenia and migraine headache using magnetic resonance imaging (MRI) Gorana Sulejmanpašić1, Enra Suljić2, Selma Šabanagić-Hajrić2 1Department of Psychiatry, 2Department of Neurology; University Clinical Center Sarajevo, Sarajevo, Bosnia and Herzegovina ABSTRACT Aim To examine the presence of morphologic variations of occi- pital sulci patternsin patients with schizophrenia and migraine he- adacheregarding gender and laterality using magnetic resonance imaging (MRI). MethodsThis study included 80 patients and brain scans were performed to analyze interhemispheric symmetry and the sulcal patterns of the occipital region of both hemispheres. Average total volumes of both hemispheres of the healthy population were used for comparison. Results There was statistically significant difference between su- bjects considering gender (p=0.012)with no difference regarding Corresponding author: age(p=0.1821). Parameters of parieto-occipital fissure (p=0.0314), Gorana Sulejmanpašić body of the calcarine sulcus (p=0.0213), inferior sagittal sulcus (p=0.0443), and lateral occipital sulcus (p=0.0411) showed stati- Department of Psychiatry, Clinical Center stically significant difference only of left hemisphere in male pati- University of Sarajevo ents with schizophrenia with shallowerdepth of the sulcus. Bolnička 25, 71000 Sarajevo, Bosnia and Herzegovina Conclusion Representation of neuroanatomical structures sugge- sts the existence of structural neuroanatomic disorders with focal Phone: +387 33 29 75 38; brain changes. Comparative analysis of occipital lobe and their Fax:+387 33 29 85 23; morphologic structures (cortical dysmorphology) in patients with E mail: [email protected] schizophreniausing MRI, according to genderindicates a signifi- cant cortical reduction in the left hemisphere only in the group of male patients compared to female patients and the control group. -

MR Evaluation of Hydrocephalus
591 MR Evaluation of Hydrocephalus Taher EI Gammal1 An analysis of sagittal T1-weighted MR studies was performed in 23 patients with Marshall B. Allen, Jr.2 hydrocephalus, 58 patients with atrophy, and 100 normal patients. The average rna mil Betty Sue Brooks 1 lopontine distance was 1.15 cm for the normal group, 1.2 cm for patients with atrophy, Edward K. Mark2 and 7.5 mm for patients with hydrocephalus. A reduction of the mamillopontine distance below 1.0 cm was found in 22 patients with hydrocephalus,S patients with atrophy, and 15 normal patients. Dilatation of the anterior third ventricle was noted in 21 patients in the hydrocephalus group and in none of the patients in the atrophy and normal groups. The average thickness of the corpus callosum at the level of the foramen of Monro was 6 mm in normal subjects and was reduced below 6 mm in 16 of the hydrocephalus patients. Smooth elevation of the corpus callosum was noted in 20 hydrocephalus patients, in 2 patients with atrophy, and in none of the normal patients. MR improves the accuracy of diagnosis in patients with hydrocephalus both because of its ability to show small obstructing lesions that are not depicted by CT and because the mass effect of the distended supratentorial ventricles produces anatomic changes that are delineated with precision by MR. CT provides incomplete information for diagnosing hydrocephalus produced by very small or isodense pathologic changes in the aqueduct and in the foramen of Monro [1, 2]. Even with advanced CT techniques, use of contrast media in the subarachnoid space and ventricular system was essential in the CT evaluation of some cases of hydrocephalus [3-5]. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

The Brain and Behavior
This page intentionally left blank The Brain and Behavior The Brain and Behavior An Introduction to Behavioral Neuroanatomy Third Edition David L. Clark Nash N. Boutros Mario F. Mendez CAMBRIDGE UNIVERSITY PRESS Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, São Paulo, Delhi, Dubai, Tokyo Cambridge University Press The Edinburgh Building, Cambridge CB2 8RU, UK Published in the United States of America by Cambridge University Press, New York www.cambridge.org Information on this title: www.cambridge.org/9780521142298 © D. Clark, N. Boutros, M. Mendez 2010 This publication is in copyright. Subject to statutory exception and to the provision of relevant collective licensing agreements, no reproduction of any part may take place without the written permission of Cambridge University Press. First published in print format 2010 ISBN-13 978-0-511-77469-0 eBook (EBL) ISBN-13 978-0-521-14229-8 Paperback Cambridge University Press has no responsibility for the persistence or accuracy of urls for external or third-party internet websites referred to in this publication, and does not guarantee that any content on such websites is, or will remain, accurate or appropriate. Every effort has been made in preparing this book to provide accurate and up-to- date information which is in accord with accepted standards and practice at the time of publication. Although case histories are drawn from actual cases, every effort has been made to disguise the identities of the individuals involved. Nevertheless, the authors, editors, and publishers can make no warranties that the information contained herein is totally free from error, not least because clinical standards are constantly changing through research and regulation. -

Graduate Neuroanatomy GSBS GS141181
Page 1 Graduate Neuroanatomy GSBS GS141181 Laboratory Guide Offered and Coordinated by the Department of Neurobiology and Anatomy The University of Texas Health Science Center at Houston. This course guide was adatped from the Medical Neuroscience Laboratory Guide. Nachum Dafny, Ph.D., Course Director; Michael Beierlein, Ph.D., Laboratory Coordinator. Online teaching materials are available at https://oac22.hsc.uth.tmc.edu/courses/neuroanatomy/ Other course information available at http://openwetware.org/wiki/Beauchamp:GraduateNeuroanatomy Contents © 2000-Present University of Texas Health Science Center at Houston. All Rights Reserved. Unauthorized use of contents subject to civil and/or criminal prosecution. Graduate Neuroanatomy : Laboratory Guide Page 2 Table of Contents Overview of the Nervous System ................................................................................................................ 3 Laboratory Exercise #1: External Anatomy of the Brain ......................................................................... 19 Laboratory Exercise #2: Internal Organization of the Brain ..................................................................... 35 Graduate Neuroanatomy : Laboratory Guide Page 3 Overview of the Nervous System Nachum Dafny, Ph.D. The human nervous system is divided into the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS, in turn, is divided into the brain and the spinal cord, which lie in the cranial cavity of the skull and the vertebral canal, respectively. The CNS and the PNS, acting in concert, integrate sensory information and control motor and cognitive functions. The Central Nervous System (CNS) The adult human brain weighs between 1200 to 1500g and contains about one trillion cells. It occupies a volume of about 1400cc - approximately 2% of the total body weight, and receives 20% of the blood, oxygen, and calories supplied to the body. The adult spinal cord is approximately 40 to 50cm long and occupies about 150cc. -
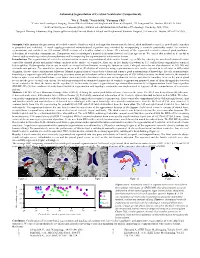
Automated Segmentation of Cerebral Ventricular Compartments
Automated Segmentation of Cerebral Ventricular Compartments 1Wu Y, 2Pohl K, 3Warfield SK, 1Cuttmann CRG. 1Center for Neurological Imaging, Harvard Medical School and Brigham and Women's Hospital, 221 Longwood Av., Boston, MA 02115 USA, 2Artificial Intelligence Laboratory,http://www.ai.mit.edu Massachusetts Institute of Technology, Cambridge MA, USA., 3Surgical Planning Laboratory http://www.spl.harvard.edu Harvard Medical School and Brigham and Women's Hospital, 75 Francis St., Boston, MA 02115 USA, Synopsis: Fully automated segmentation of cerebral ventricle chambers, which is designed to discriminate the lateral, third and fourth ventricles, as well as the aqueduct is presented and validated. A novel topology-restricted intensity-based algorithm was extended by incorporating a ventricle probability model for ventricle segmentation, and validated on 124 coronal SPGR sections of a healthy volunteer’s brain. 3D-rendering of the segmented ventricles showed good qualitative delineation of ventricular compartments. Comparison with a radiologist’s manual delineation showed excellent agreement. We expect this method to be useful in clinical studies involving ventricular morphometry and for improving the segmentation of white matter lesions. Introduction: The segmentation of ventricles is important for accurate segmentation of white matter lesions, e.g. in MS. by reducing the misclassification of lesions caused by choroid plexus and partial volume artifacts at the surface of ventricles. Also, one in five hundred newborn in U.S. suffers from congenital or acquired hydrocephalus. Hydrocephalus also occurs in adults as a result of head trauma, meningitis, tumors or cysts. Enlarged ventricles are also observed in AD, MS and schizophrenia patients. The quantitative measurement, as well as 3D display of ventricles using segmentation in vivo can be expected to be of value in differential diagnosis, disease characterization and follow-up.