Comparative Total Syntheses of Strychnine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis of FMN Analogues As Probes for the Photocycle of Avena Sativa
Synthesis of FMN Analogues as Probes for the Photocycle of Avena sativa Andrew Wood Submitted in fulfilment of the requirement for the degree of Doctor of Philosophy School of Chemistry, Cardiff University February, 2014 Declaration This work has not been submitted in substance for any other degree or award at this or any other university or place of learning, nor is being submitted concurrently in candidature for any degree or other award. Signed ………………………………………… (candidate) Date ………………………… STATEMENT 1 This thesis is being submitted in partial fulfilment of the requirements for the degree of PhD. Signed ………………………………………… (candidate) Date ………………………… STATEMENT 2 This thesis is the result of my own independent work/investigation, except where otherwise stated (see below). Other sources are acknowledged by explicit references. The views expressed are my own. Signed ………………………………………… (candidate) Date ………………………… STATEMENT 3 I hereby give consent for my thesis, if accepted, to be available for photocopying and for inter- library loan, and for the title and summary to be made available to outside organisations. Signed ………………………………………… (candidate) Date ………………………… i Declaration of Contributions I confirm that the work presented within this thesis was performed by the author, with exceptions summarised here, and explicitly referenced in the relevant sections of the thesis. The original construction of several plasmid vectors used for the expression of recombinant proteins was not performed, as they were freely available (from previous work), and donated to me. Their original preparation is summarised below. Furthermore, during a short side- project the dephosphorylation of 5-deazaFAD was performed (on an analytical scale) by a collaborator (Dr. Hannah Collins, University of Kent) using commercially available Phosphodiesterase I from Crotalus atrox. -

Abstract Multicomponent Reactions of Salicylaldehyde, Cyclic Ketones, and Arylamines Through Cooperative Enamine-Metal Lewis
ABSTRACT MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS by Ryan Gregory Sarkisian Multicomponent reactions (MCRs) are the most atom economic, highly selective, and convergent type of reaction. This allows for a reaction to have a wide scope and allows for maximization of the complexity of a product. Catalyzing these MCRs with asymmetric catalysis is a novel way to introduce stereocontrol into highly complex molecules with various functional groups. Asymmetric catalysis is considered the most efficient method for constructing highly functionalized optically active stereopure compounds. There are three pillars of asymmetric catalysis: biocatalysis, transition metal catalysis, and organocatalysis. This research focuses on two of these pillars, transition metal catalysis and organocatalysis, working cooperatively to catalyze this MCR. The focus is to educate or refresh the audience on the basic topics that make up the complexity of the MCRs being catalyzed by cooperative asymmetric catalysis. Ultimately to explore the cooperative catalysts used to synthesize both the racemic and asymmetric three fused ring products (9-((4-methoxyphenyl)amino)-2,3,4,4a,9,9a-hexahydro-1H-xanthen-4a-ol). MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS A THESIS Submitted to the Faculty of Miami University in partial fulfillment of the requirements for the degree of Master of Science Department of -

Catalytic Direct Asymmetric Michael Reactions
ORGANIC LETTERS 2001 Catalytic Direct Asymmetric Michael Vol. 3, No. 23 Reactions: Taming Naked Aldehyde 3737-3740 Donors Juan M. Betancort and Carlos F. Barbas III* The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 [email protected] Received September 5, 2001 ABSTRACT Direct catalytic enantio- and diastereoselective Michael addition reactions of unmodified aldehydes to nitro olefins using (S)-2-(morpholinomethyl)- pyrrolidine as a catalyst are described. The reactions proceed in good yield (up to 96%) in a highly syn-selective manner (up to 98:2) with enantioselectivities approaching 80%. The resulting γ-formyl nitro compounds are readily converted to chiral, nonracemic 3,4-disubstituted pyrrolidines. The Michael reaction is generally regarded as one of the Typically, carbon nucleophiles that contain an active most efficient carbon-carbon bond forming reactions, and methylene center such as malonic acid esters, â-keto esters, studies concerning this reaction have played an important nitroalkanes, etc. have been studied in the Michael reaction. role in the development of modern synthetic organic Carbonyl compounds, and ketones in particular, have gener- chemistry.1 As the demand for optically active compounds ally only been used as donors following their preactivation has soared in recent years, much progress has been made in by conversion into a more reactive species such as enol or the development of asymmetric variants of this reaction, enamine equivalents.5,6 In these cases, additional synthetic providing for the preparation of Michael adducts with high enantiomeric purity.2 Though remarkable advances have been (3) (a) Chataigner, I.; Gennari, C.; Ongeri, S.; Piarulli, U.; Ceccarelli, S. -

A General and Direct Reductive Amination of Aldehydes and Ketones with Electron-Deficient Anilines
SYNTHESIS0039-78811437-210X © Georg Thieme Verlag Stuttgart · New York 2016, 48, 1301–1317 1301 feature Syn thesis J. Pletz et al. Feature A General and Direct Reductive Amination of Aldehydes and Ketones with Electron-Deficient Anilines Jakob Pletz EWG hydride reductant EWG H NH 1 N R1 Bernhard Berg 2 O R activating agent + Rolf Breinbauer* 2 R2 R Institute of Organic Chemistry, Graz University of Technology, 29 examples up to 99% yield Stremayrgasse 9, 8010 Graz, Austria 3 methods: [email protected] A) BH3•THF, AcOH, CH2Cl2, 0–20 °C 12 anilines In memoriam Philipp Köck B) BH3•THF, TMSCl, DMF, 0 °C 14 ketones C) NaBH4, TMSCl, DMF, 0 °C 3 aldehydes Received: 01.12.2015 Our first goal was the design and synthesis of analogues Accepted after revision: 20.01.2016 of intermediates in the transformation catalyzed by the Published online: 01.03.2016 1 DOI: 10.1055/s-0035-1561384; Art ID: ss-2015-t0688-fa protein PhzA/B. According to Wulf’s proposal, this enzyme was expected to catalyze the imine formation between the putative aminoketone B, resulting from the transformation Abstract In our ongoing efforts in preparing tool compounds for in- of PhzF with dihydrohydroxyanthranilic acid (DHHA; A), vestigating and controlling the biosynthesis of phenazines, we recog- with itself, thereby establishing the tricyclic skeleton C, nized the limitations of existing protocols for C–N bond formation of from which after a series of oxidation reactions phenazine- electron-deficient anilines when using reductive amination. After ex- carboxylic acid E will -

Copyrighted Material
Index Abbreviations receptor sites 202, 211 weak 122 amino acids, Table 500–1 muscarinic 413 weak, pH calculation 147–8 nucleic acids 551 nicotinic 413 Acid–base nucleotides, Table 551 as quaternary ammonium salt 202 catalysis, enzymes 516 peptides and proteins 504 Acetylcholinesterase equilibria 121 phosphates and diphosphates 277 enzyme mechanism 519–21 interactions, predicting 155–7 structural, Table 14 hydrolysis of acetylcholine 279 Acidic reagents, Table 157 ACE (angiotensin-converting enzyme) inhibitors 279 Acidity enzyme action 532 Acetyl-CoA carboxylase, in fatty acid acidity constant 122 inhibitors 532 biosynthesis 595 bond energy effects 125 Acetal Acetyl-CoA (acetyl coenzyme A) definition 121 in etoposide 233 as acylating agent 262 electronegativity effects 125 formation 229 carboxylation to malonyl-CoA 595, hybridization effects 128 polysaccharides as polyacetals 232 609 inductive effects 125–7 as protecting group 230 Claisen reaction 381 influence of electronic and structural Acetal and ketal enolate anions 373 features 125–34 cyclic, as protecting groups 481 in Krebs cycle 585 and leaving groups, Table 189 groups in sucrose 231 from β-oxidation of fatty acids 388 pKa values 122–5 Acetaldehyde, basicity 139 as thioester 262, 373 resonance / delocalization effects 129–34 Acetamide, basicity 139 Acetylene, bonding molecular orbitals 31 Acidity (compounds) Acetaminophen, see paracetamol Acetylenes, acidity 128 acetone 130 Acetoacetyl-CoA, biosynthesis from N-Acetylgalactosamine, in blood group acetonitrile 365 acetyl-CoA 392 -

Recommended Methods for the Identification and Analysis Of
Vienna International Centre, P.O. Box 500, 1400 Vienna, Austria Tel: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES Laboratory and Scientific Section United Nations Office on Drugs and Crime Vienna RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES UNITED NATIONS New York, 2006 Note Mention of company names and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. ST/NAR/34 UNITED NATIONS PUBLICATION Sales No. E.06.XI.1 ISBN 92-1-148208-9 Acknowledgements UNODC’s Laboratory and Scientific Section wishes to express its thanks to the experts who participated in the Consultative Meeting on “The Review of Methods for the Identification and Analysis of Amphetamine-type Stimulants (ATS) and Their Ring-substituted Analogues in Seized Material” for their contribution to the contents of this manual. Ms. Rosa Alis Rodríguez, Laboratorio de Drogas y Sanidad de Baleares, Palma de Mallorca, Spain Dr. Hans Bergkvist, SKL—National Laboratory of Forensic Science, Linköping, Sweden Ms. Warank Boonchuay, Division of Narcotics Analysis, Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand Dr. Rainer Dahlenburg, Bundeskriminalamt/KT34, Wiesbaden, Germany Mr. Adrian V. Kemmenoe, The Forensic Science Service, Birmingham Laboratory, Birmingham, United Kingdom Dr. Tohru Kishi, National Research Institute of Police Science, Chiba, Japan Dr. -
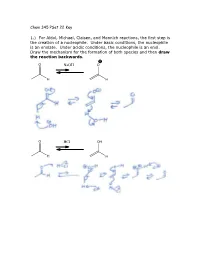
Chem 345 Pset 22 Key 1.) for Aldol, Michael, Claisen, and Mannich
Chem 345 PSet 22 Key 1.) For Aldol, Michael, Claisen, and Mannich reactions, the first step is the creation of a nucleophile. Under basic conditions, the nucleophile is an enolate. Under acidic conditions, the nucleophile is an enol. Draw the mechanism for the formation of both species and then draw the reaction backwards. O NaOH O H H O HCl OH H H 2.) In the case of a Mannich reaction, the electrophile (an imine) must also be made. Draw the mechanism for the forward and backward reaction: O MeNH2 N H H cat. AcOH H H 3.) When a secondary amine is used and the carbonyl has an enolizable proton, an enamine can be formed. Are enamines nucleophilic or electrophilic? Nucleophilic H O N N H2O H cat. AcOH H enamine N N H H enamine 4.) The following reaction is the Lobry-de-Bruyn-von-Ekenstein reaction. Don’t worry about the name. I only recently found out that that was the official name for the rearrangement leading from glucose to fructose or mannose and back again. I was taught it under a different name: the Carbohydrate Game. Under acidic or basic conditions, it is possible that the following sugars (glucose, fructose, and mannose) are in equilibrium with each other. Draw the acid catalyzed version and the base catalyzed version. (Hint: this is just a repeat of question 1.) O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose The sugars are drawn in a Fisher projection (or what I think of is a dead cat projection). -

Heterocyclic Chemistrychemistry
HeterocyclicHeterocyclic ChemistryChemistry Professor J. Stephen Clark Room C4-04 Email: [email protected] 2011 –2012 1 http://www.chem.gla.ac.uk/staff/stephenc/UndergraduateTeaching.html Recommended Reading • Heterocyclic Chemistry – J. A. Joule, K. Mills and G. F. Smith • Heterocyclic Chemistry (Oxford Primer Series) – T. Gilchrist • Aromatic Heterocyclic Chemistry – D. T. Davies 2 Course Summary Introduction • Definition of terms and classification of heterocycles • Functional group chemistry: imines, enamines, acetals, enols, and sulfur-containing groups Intermediates used for the construction of aromatic heterocycles • Synthesis of aromatic heterocycles • Carbon–heteroatom bond formation and choice of oxidation state • Examples of commonly used strategies for heterocycle synthesis Pyridines • General properties, electronic structure • Synthesis of pyridines • Electrophilic substitution of pyridines • Nucleophilic substitution of pyridines • Metallation of pyridines Pyridine derivatives • Structure and reactivity of oxy-pyridines, alkyl pyridines, pyridinium salts, and pyridine N-oxides Quinolines and isoquinolines • General properties and reactivity compared to pyridine • Electrophilic and nucleophilic substitution quinolines and isoquinolines 3 • General methods used for the synthesis of quinolines and isoquinolines Course Summary (cont) Five-membered aromatic heterocycles • General properties, structure and reactivity of pyrroles, furans and thiophenes • Methods and strategies for the synthesis of five-membered heteroaromatics -

Mannich Reaction
1. Introduction 1.1- Mannich Reaction The Mannich reaction is three component condensation in which a compound containing an active hydrogen atom is allowed to react with formaldehyde and an NH-amine derivative . Secondary amines rather than primary amines and ammonia are employed , the resulting product (Mannich Base ) is an amine compound having the N atom linked to the R substrate through a methylene group 1,2. The aminoalkylation of CH-acidic compounds was described by several authors as early as the 19th. century. However, it was Carl Mannich who was the first to recognize the enormous significance of this reaction , and it was he who extended the chemistry into a broad based synthetic methodology through systematic research. Since then this reaction that now carries his name has developed into one of the most important C-C bond-forming reactions in organic chemistry3,4. The Mannich reaction is a classical method for the preparation of β -aminoketones and aldehydes (Mannich bases) and, as such, is one of the most important basic reaction types in organic chemistry. It is the key step in the synthesis of numerous pharmaceuticals and natural products3,4. 1 The Mannich reaction can be presented by the following equation: The essential feature of the reaction is the replacement of the active hydrogen atom by an aminomethyl or substituted aminomethyl group. The symbolizes the active hydrogen component which includes ketones, aldehydes, acids, esters, phenols, acetylenes, picolines, nitroalkanes and quinolines1,2. 1.2- Mechanism of the Mannich reaction The mechanism of the Mannich reaction has been well investigated, the condensation reaction occurs in two steps: first, the amine reacts with formaldehyde to give condensation product (5 ), (6), ( 7) (step I) which then attacks the substrate R-H (step II) .The reaction does not normally follow the other possible route (step III and IV) ; however, some successful reaction between hydroxymethyl derivatives (8) and alkylamines to give Mannich bases (9) should be mentioned . -

Simple Ruthenium-Catalyzed Reductive Amination Enables the Synthesis of a Broad Range of Primary Amines
ARTICLE DOI: 10.1038/s41467-018-06416-6 OPEN Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines Thirusangumurugan Senthamarai1, Kathiravan Murugesan1, Jacob Schneidewind 1, Narayana V. Kalevaru1, Wolfgang Baumann1, Helfried Neumann1, Paul C.J. Kamer1, Matthias Beller 1 & Rajenahally V. Jagadeesh 1 1234567890():,; The production of primary benzylic and aliphatic amines, which represent essential feed- stocks and key intermediates for valuable chemicals, life science molecules and materials, is of central importance. Here, we report the synthesis of this class of amines starting from carbonyl compounds and ammonia by Ru-catalyzed reductive amination using H2. Key to success for this synthesis is the use of a simple RuCl2(PPh3)3 catalyst that empowers the synthesis of >90 various linear and branched benzylic, heterocyclic, and aliphatic amines under industrially viable and scalable conditions. Applying this catalyst, −NH2 moiety has been introduced in functionalized and structurally diverse compounds, steroid derivatives and pharmaceuticals. Noteworthy, the synthetic utility of this Ru-catalyzed amination protocol has been demonstrated by upscaling the reactions up to 10 gram-scale syntheses. Further- more, in situ NMR studies were performed for the identification of active catalytic species. Based on these studies a mechanism for Ru-catalyzed reductive amination is proposed. 1 Leibniz-Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock, Germany. Correspondence and requests for materials should be addressed to M.B. (email: [email protected]) or to R.V.J. (email: [email protected]) NATURE COMMUNICATIONS | (2018) 9:4123 | DOI: 10.1038/s41467-018-06416-6 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-018-06416-6 fi 25 he development of ef cient catalytic reactions for the Cl]2-BINAS catalysts were also applied . -

Enantioselective Organocatalytic Reductive Amination R
Published on Web 12/14/2005 Enantioselective Organocatalytic Reductive Amination R. Ian Storer, Diane E. Carrera, Yike Ni, and David W. C. MacMillan* DiVision of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, California 91125 Received October 23, 2005; E-mail: [email protected] The reductive amination reaction remains one of the most ketone and amine coupling partners to a chiral hydrogen-bonding powerful and widely utilized transformations available to practi- catalyst8 would result in the intermediate formation of an iminium tioners of chemical synthesis in the modern era.1 A versatile species that in the presence of a suitable Hantzsch ester would coupling reaction that enables the chemoselective union of diverse undergo enantioselective hydride reduction, thereby allowing asym- ketone and amine containing fragments, reductive amination can metric reductive amination in an in vitro setting.9 This proposal also provide rapid and general access to stereogenic C-N bonds, was further substantiated by the significant advances in hydrogen- a mainstay synthon found in natural isolates and medicinal agents. bonding catalysis, arising from the pioneering studies of Jacobsen,10 While a variety of protocols have been described for the asymmetric Corey,11 Takemoto,12 Rawal,13 Johnston,14 Akiyama,15 and Terada.16 reduction of ketimines (a strategy that requires access to preformed, bench stable imines),2 it is surprising that few laboratory methods are known for enantioselective reductive amination.1b,3 Moreover, the use of this ubiquitous reaction for the union of complex fragments remains unprecedented in the realm of asymmetric catalysis, a remarkable fact given the widespread application of both racemic and diastereoselective variants. -

Convenient Reductive Amination of Aldehydes by Nabh4/Cation Exchange Resin
J. Mex. Chem. Soc. 2014, 58(1), 22-26 Article22 J. Mex. Chem. Soc. 2014, 58(1) Davood© Setamdideh2014, Sociedad and FarhadQuímica Sepehraddin de México ISSN 1870-249X Convenient Reductive Amination of Aldehydes by NaBH4/Cation Exchange Resin Davood Setamdideh* and Farhad Sepehraddin Department of Chemistry, College of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, Iran. [email protected]; [email protected] Received June 10th, 2013; Accepted September 18th, 2013 Abstract. Different secondary amines have been synthesized by re- Resumen. Se sintetizaron diversas aminas secundarias por aminación ductive amination a variety of aldehydes and anilines with NaBH4/ reductiva de una variedad de aldehidos y anilinas empleando como DOWEX(R)50WX8 as reducing system in THF at room temperature sistema reductor NaBH4/DOWEX(R)50WX8 en THF a temperatura in high to excellent yields of products (85-93%). ambiente con rendimientos de altos a excelentes. Key words: NaBH4, DOWEX(R)50WX8, Reductive amination, Car- Palabras clave: NaBH4, DOWEX(R)50WX8, aminación reductiva, bonyl compounds. aminas secundarias. Introduction Results and Discussion Amines are important in drugs and in active pharmaceuti- Recently, we have reported that the DOWEX(R)50WX4 (low cal intermediates. Amines can be achieved by reduction of price cation exchange resin, strong acid) can be used as recy- nitro, cyano, azide, carboxamide derivatives or alkylation of clable catalyst for the regioselective synthesis of Oximes by amines (using alkyl halides or sulfonates). On the other hand, NH2OH.HCl/DOWEX(R)50WX4 system [31], reduction of a direct reductive amination (DRA) is other approach which of- variety of carbonyl compounds such as aldehydes, ketones, α- fers significant advantages.