Mannich Reaction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fluorescent Aminal Linked Porous Organic Polymer for Reversible Iodine Capture and Sensing Muhammad A
www.nature.com/scientificreports OPEN Fluorescent aminal linked porous organic polymer for reversible iodine capture and sensing Muhammad A. Sabri1, Mohammad H. Al‑Sayah2, Susan Sen2, Taleb H. Ibrahim1 & Oussama M. El‑Kadri2* A novel triazene-anthracene-based fuorescent aminal linked porous organic polymer (TALPOP) was prepared via metal free-Schif base polycondensation reaction of 9,10-bis-(4,6-diamino-S‑triazin‑ 2-yl)anthracene and 2-furaldehyde. The polymer has exceptional chemical and thermal stabilities and exhibit good porosity with Brunauer–Emmett–Teller surface area of 401 m2g−1. The combination of such porosity along with the highly conjugated heteroatom-rich framework enabled the polymer to exhibit exceptional iodine vapor uptake of up to 314 wt % and reversible iodine adsorption in solution. Because of the inclusion of the anthracene moieties, the TALPOP exhibited excellent 3 −1 detection sensitivity towards iodine via forescence quenching with Ksv value of 2.9 × 10 L mol . The cost efective TALPOP along with its high uptake and sensing of iodine, make it an ideal material for environmental remediation. Nuclear energy is becoming one of the most feasible alternative sources to meet the ever-increasing energy demand and minimize the emission of greenhouse gases because of its high-density energy, minimal carbon footprints, and low operation cost1–4. Despite such advantages, the potential emissions of radioactive material 129 131 3 14 85 (such as I and I, H, CO2, and Kr) from nuclear energy power plants is a major drawback of this tech- nology due to the serious environmental and health efect of these materials4,5. -

New Insights Into the Mechanism of Schiff Base Synthesis from Aromatic
A peer-reviewed version of this preprint was published in PeerJ on 15 December 2020. View the peer-reviewed version (peerj.com/articles/ochem-4), which is the preferred citable publication unless you specifically need to cite this preprint. Silva PJ. 2020. New insights into the mechanism of Schiff base synthesis from aromatic amines in the absence of acid catalyst or polar solvents. PeerJ Organic Chemistry 2:e4 https://doi.org/10.7717/peerj-ochem.4 New insights into the mechanism of Schiff base synthesis from aromatic amines in the absence of acid catalyst or polar solvents Pedro J Silva Corresp. 1 1 FP-ENAS/Fac. de Ciências da Saúde, Universidade Fernando Pessoa, Porto, Portugal Corresponding Author: Pedro J Silva Email address: [email protected] Extensive computational studies of the imine synthesis from amines and aldehydes in water have shown that the large-scale structure of water is needed to afford appropriate charge delocalisation and enable sufficient transition state stabilisation. These insights cannot, however, be applied to the understanding of the reaction pathway in apolar solvents due their inability to form extensive hidrogen-bonding networks. In this work, we perform the first computational studies of this reaction in apolar conditions. This density- functional study of the reaction of benzaldehyde with four closely related aromatic amines (aniline, o-toluidine, m-toluidine and p-toluidine) shows that an additional molecule of amine may provide enough stabilization of the first transition state even in the absence of a hydrogen bonding network. Our computations also show that the second reaction step cannot take place unless an extra proton is added to the system but, crucially, that reaction rate is so high that even picomolar amounts of protonated base are enough to achieve realistic rates. -

Abstract Multicomponent Reactions of Salicylaldehyde, Cyclic Ketones, and Arylamines Through Cooperative Enamine-Metal Lewis
ABSTRACT MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS by Ryan Gregory Sarkisian Multicomponent reactions (MCRs) are the most atom economic, highly selective, and convergent type of reaction. This allows for a reaction to have a wide scope and allows for maximization of the complexity of a product. Catalyzing these MCRs with asymmetric catalysis is a novel way to introduce stereocontrol into highly complex molecules with various functional groups. Asymmetric catalysis is considered the most efficient method for constructing highly functionalized optically active stereopure compounds. There are three pillars of asymmetric catalysis: biocatalysis, transition metal catalysis, and organocatalysis. This research focuses on two of these pillars, transition metal catalysis and organocatalysis, working cooperatively to catalyze this MCR. The focus is to educate or refresh the audience on the basic topics that make up the complexity of the MCRs being catalyzed by cooperative asymmetric catalysis. Ultimately to explore the cooperative catalysts used to synthesize both the racemic and asymmetric three fused ring products (9-((4-methoxyphenyl)amino)-2,3,4,4a,9,9a-hexahydro-1H-xanthen-4a-ol). MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS A THESIS Submitted to the Faculty of Miami University in partial fulfillment of the requirements for the degree of Master of Science Department of -

Catalytic Direct Asymmetric Michael Reactions
ORGANIC LETTERS 2001 Catalytic Direct Asymmetric Michael Vol. 3, No. 23 Reactions: Taming Naked Aldehyde 3737-3740 Donors Juan M. Betancort and Carlos F. Barbas III* The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 [email protected] Received September 5, 2001 ABSTRACT Direct catalytic enantio- and diastereoselective Michael addition reactions of unmodified aldehydes to nitro olefins using (S)-2-(morpholinomethyl)- pyrrolidine as a catalyst are described. The reactions proceed in good yield (up to 96%) in a highly syn-selective manner (up to 98:2) with enantioselectivities approaching 80%. The resulting γ-formyl nitro compounds are readily converted to chiral, nonracemic 3,4-disubstituted pyrrolidines. The Michael reaction is generally regarded as one of the Typically, carbon nucleophiles that contain an active most efficient carbon-carbon bond forming reactions, and methylene center such as malonic acid esters, â-keto esters, studies concerning this reaction have played an important nitroalkanes, etc. have been studied in the Michael reaction. role in the development of modern synthetic organic Carbonyl compounds, and ketones in particular, have gener- chemistry.1 As the demand for optically active compounds ally only been used as donors following their preactivation has soared in recent years, much progress has been made in by conversion into a more reactive species such as enol or the development of asymmetric variants of this reaction, enamine equivalents.5,6 In these cases, additional synthetic providing for the preparation of Michael adducts with high enantiomeric purity.2 Though remarkable advances have been (3) (a) Chataigner, I.; Gennari, C.; Ongeri, S.; Piarulli, U.; Ceccarelli, S. -

Study of Reactions of Two Mannich Bases Derived of 4’-Hydroxychalcones with Glutathione by RP‑TLC, RP‑HPLC and RP‑HPLC‑ESI‑MS Analysis
http://dx.doi.org/10.21577/0103-5053.20160260 J. Braz. Chem. Soc., Vol. 28, No. 6, 1048-1062, 2017. Printed in Brazil - ©2017 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00 Article Study of Reactions of Two Mannich Bases Derived of 4’-Hydroxychalcones with Glutathione by RP-TLC, RP-HPLC and RP-HPLC-ESI-MS Analysis Aline Bernardes,a,b Caridad N. Pérez,a Mátyás Mayer,c Cameron C. da Silva,a Felipe T. Martinsa and Pál Perjési*,b aInstituto de Química, Universidade Federal de Goiás, 74690-900 Goiânia-GO, Brazil bInstitute of Pharmaceutical Chemistry and cDepartment of Forensic Medicine, University of Pécs, H-7624 Pécs, Hungary 4’-Hydroxychalcones have been reported to possess several beneficial biological effects. Several lines of evidence accumulated to demonstrate increased biological activities of the Mannich base derivatives of the parent 4’-hydroxychalcones. Bioactivities of chalcones and related α,β-unsaturated ketones are frequently associated with their reactivity with cellular thiols, such as GSH. For comparison of GSH reactivity, two bis Mannich bases of two 4’-hydroxychalcones were synthesized and reacted with GSH under non-cellular conditions. Reversed-phase thin layer chromatography (RP-TLC) and reversed-phase high performance liquid chromatography (RP-HPLC) analysis showed formation of two polar products which structures were confirmed by RP-HPLC-ESI-MS (RP-HPLC-electrospray ionization mass spectrometry) as 1:1 chalcone-GSH adducts in each case. At pH values below 8.0, the two bis Mannich bases showed higher GSH reactivity than two 4’-hydroxychalcones. Influence of the nature of the amino groups, the ring-B substituents and pH of the medium on reactivity was also investigated. -

Research Journal of Pharmaceutical, Biological and Chemical Sciences
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Metal Complexes of Schiff's Bases Containing Sulfonamides Nucleus: A Review. Zainab Hussain1, Majid Khalaf2, Hadeel Adil2, Dheaa Zageer2, 3, Firas Hassan2, Salam Mohammed4, and Emad Yousif1*. 1Department of Chemistry, College of Science, Misan University, Misan, Iraq . 2Department of Chemistry, College of Science, Al-Nahrain University, Baghdad, Iraq. 3Forensic DNA Center for Research and Training, Al-Nahrain University, Baghdad, Iraq. 4College of Engineering and Architecture, University of Nizwa, Birkat Almouz, 616 Nizwa, Oman. ABSTRACT Schiff's bases are versatile ligands which are synthesized from the condensation of primary amines with carbonyl groups. Schiff's bases play an important role in Inorganic chemistry due to formation of very stable complexes with various transition and inner-Transition Metals. Transition metal complexes derived from the Schiff base ligands have widly applications. This review summarizes some metal complexes of schiff's bases derived from sulfonamide derivatives and reviewed the applications of Schiff's bases chelates in quantitative analysis. Keywords: Schiff bases, Metal complexes, Quantitative analysis. *Corresponding author September – October 2016 RJPBCS 7(5) Page No. 1008 ISSN: 0975-8585 Overview of of Schiff's bases A Schiff's base is a nitrogen analog of an aldehyde or ketone in which the C=O group is replaced by C=N-R group. It is usually formed by condensation of an aldehyde or ketone with a primary amine according to the following scheme (Scheme 1): Scheme 1: Formation of Schiff's bases. Where R, may be an alkyl or an aryl group. Schiff's bases that contain aryl substituents are substantially more stable and more readily synthesized, while those which contain alkyl substituents are relatively unstable. -

Copyrighted Material
Index Abbreviations receptor sites 202, 211 weak 122 amino acids, Table 500–1 muscarinic 413 weak, pH calculation 147–8 nucleic acids 551 nicotinic 413 Acid–base nucleotides, Table 551 as quaternary ammonium salt 202 catalysis, enzymes 516 peptides and proteins 504 Acetylcholinesterase equilibria 121 phosphates and diphosphates 277 enzyme mechanism 519–21 interactions, predicting 155–7 structural, Table 14 hydrolysis of acetylcholine 279 Acidic reagents, Table 157 ACE (angiotensin-converting enzyme) inhibitors 279 Acidity enzyme action 532 Acetyl-CoA carboxylase, in fatty acid acidity constant 122 inhibitors 532 biosynthesis 595 bond energy effects 125 Acetal Acetyl-CoA (acetyl coenzyme A) definition 121 in etoposide 233 as acylating agent 262 electronegativity effects 125 formation 229 carboxylation to malonyl-CoA 595, hybridization effects 128 polysaccharides as polyacetals 232 609 inductive effects 125–7 as protecting group 230 Claisen reaction 381 influence of electronic and structural Acetal and ketal enolate anions 373 features 125–34 cyclic, as protecting groups 481 in Krebs cycle 585 and leaving groups, Table 189 groups in sucrose 231 from β-oxidation of fatty acids 388 pKa values 122–5 Acetaldehyde, basicity 139 as thioester 262, 373 resonance / delocalization effects 129–34 Acetamide, basicity 139 Acetylene, bonding molecular orbitals 31 Acidity (compounds) Acetaminophen, see paracetamol Acetylenes, acidity 128 acetone 130 Acetoacetyl-CoA, biosynthesis from N-Acetylgalactosamine, in blood group acetonitrile 365 acetyl-CoA 392 -

Synthesis and Characterization of Polymeric Schiff Bases
SYNTHESIS AND CHARACTERIZATION OF POLYMERIC SCHIFF BASES FROM 2,5-DIFORMYLFURAN A Thesis Present to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Tengfei Xiang December, 2012 SYNTHESIS AND CHARACTERIZATION OF POLYMERIC SCHIFF BASES FROM 2,5-DIFORMYLFURAN Tengfei Xiang Thesis Approved: Accepted: Advisor Dean of the College Dr. Yi Pang Dr. Chand Midha Co-Advisor Dean of the Graduate School Dr. Ping Yi Dr. George R. Newkome Department Chair Date Dr. Kim C. Calvo ii ABSTRACT Furan derivatives have the potential to be an alternative resource since it can be obtained from biomass in large scale. Many monomers derived from furfural and 5-hydroxymethylfurfural (HMF), have been used in polymerization investigation in recent years. However, few studies in using 2,5-diformylfuran(DFF) as monomer has been reported, probably due to the limited availability of monmer. Polymeric Schiff bases (or polyimines) is a class of materials containing –CH=N structural unit, which exhibit good thermal stability, useful mechanical properties. In this work, the polymeric Schiff bases obtained from polycondensation of DFF and aromatic, aliphatic diamines, and more comprehensive characterization study has been carried out. FT-IR and 13C Solid NMR study of products confirmed the formation of – CH=N structural unit. And MALDI-TOF mass spectrum showed a typical polymer pattern, providing further understanding on the polymerization process. Thermal Analysis had also been carried out for both alphatic and aromatic polymeric Schiff Bases. iii DEDICATION I would like to dedicate this thesis to my mother, Yumei Jiang, for her love, patience and understanding. -

Generation of a Structurally Diverse Library Through Alkylation and Ring
70 Acta Chim. Slov. 2013, 60, 70–80 Scientific paper Generation of a Structurally Diverse Library through Alkylation and Ring Closure Reactions Using 3-Dimethylamino-1-(thiophen-2-yl)propan-1-one Hydrochloride* Gheorghe Roman Petru Poni Institute of Macromolecular Chemistry, 41A Aleea Gr. Ghica Vodâ, Ias¸i 700487, Romania Corresponding author: E-mail: [email protected] Received: 01-06-2012 Abstract 3-Dimethylamino-1-(thiophen-2-yl)propan-1-one hydrochloride (2), a ketonic Mannich base derived from 2-acetyl- thiophene, was used as a starting material in different types of alkylation and ring closure reactions with a view to gene- rate a structurally diverse library of compounds. Compound 2 reacts with S-alkylated dithiocarbamic acid salts and aryl mercaptans to produce dithiocarbamates and thioethers, respectively. The dimethylamino moiety in compound 2 was exchanged with various aliphatic secondary and aromatic primary and secondary amines, whereas monocyclic NH-azoles such as pyrazole, imidazole, 1,2,4-triazole, and tetrazole were N-alkylated by compound 2. Ketones, pyrrole and in- doles have been the substrates subjected to C-alkylation reactions by compound 2. Ring closure reactions of compound 2 with a suitable bifunctional nucleophile yielded pyrazolines, pyridines, 2,3-dihydro-1,5-1H-benzodiazepines, 2,3- dihydro-1,5-1H-benzothiazepine, pyrimido[1,2-a]benzimidazole and 4-hydroxypiperidine derivatives. Keywords: Ketonic Mannich base, alkylation, amine exchange, cyclization. 1. Introduction through the replacement of the easily leaving dimethyla- mino group by various nucleophiles. Also, several cycliza- The chemistry of Mannich bases has drawn a great tions of the aforementioned Mannich base with bifunctio- deal of attention owing to the high synthetic potential and nal nucleophiles to 5-, 6- and 7-membered nitrogen-con- the outstanding applications of this class of compounds.2–4 taining heterocycles have been investigated. -

Comparative Total Syntheses of Strychnine
Comparative Total Syntheses of Strychnine N C D MacMillan Group Meeting N H O H A H B E Nathan Jui H N N H H F G July 22, 2009 O O O References: Pre-Volhardt: Bonjoch Chem. Rev. 2000, 3455. Woodward Tetrahedron, 1963, 247. Volhardt J. Am. Chem. Soc. 2001, 9324. Magnus J. Am. Chem. Soc. 1993, 8116. Martin J. Am. Chem. Soc. 2001, 8003. Overman J. Am. Chem. Soc. 1995, 5776. Bodwell Angew. Chem. Int. Ed. 2002, 3261. Kuehne J. Org. Chem. 1993, 7490. Mori J. Am. Chem. Soc. 2003, 9801. Kuehne J. Org. Chem. 1998, 9427. Shibasaki Tetrahedron 2004, 9569. Rawal J. Org. Chem. 1994, 2685. Fukuyama J. Am. Chem. Soc. 2004, 10246. Bosch, Bonjoch Chem. Eur. J. 2000, 655. Padwa Org. Lett. 2007, 279. History and Structure of (!)-Strychnine ! Isolated in pure form in 1818 (Pelletier and Caventou) ! Structural Determination in 1947 (Robinson and Leuchs) ! 24 skeletal atoms (C21H22N2O2) ! Isolated in pure form in 1818 (Pelletier and Caventou) N ! Over !25 07 -pruinbglisc,a 6ti-osntesr epoecrteaninteinrgs to structure ! Structural Determination in 1947 (Robinson and Leuchs) ! Notor!io u Ss ptoirxoicne (nletethr a(lC d-o7s) e ~10-50 mg / adult) N H H ! Over 250 publications pertaining to structure O O ! Comm!o nClyD uEs eridn gr osdyesntet mpoison ! Notorious poison (lethal dose ~10-50 mg / adult) ! Hydroxyethylidine Strychnos nux vomica ! $20.20 / 10 g (Aldrich), ~1.5 wt% (seeds), ~1% (blossoms) "For it's molecular size it is the most complex substance known." -Robert Robinson History and Structure of (!)-Strychnine ! 24 skeletal atoms (C21H22N2O2) N ! 7-rings, 6-stereocenters ! Spirocenter (C-7) N H H O O ! CDE ring system ! Hydroxyethylidine "For it's molecular size it is the most complex substance known." -Robert Robinson "If we can't make strychnine, we'll take strychnine" -R. -
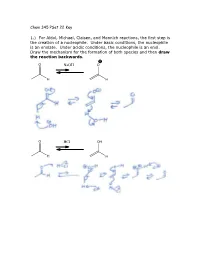
Chem 345 Pset 22 Key 1.) for Aldol, Michael, Claisen, and Mannich
Chem 345 PSet 22 Key 1.) For Aldol, Michael, Claisen, and Mannich reactions, the first step is the creation of a nucleophile. Under basic conditions, the nucleophile is an enolate. Under acidic conditions, the nucleophile is an enol. Draw the mechanism for the formation of both species and then draw the reaction backwards. O NaOH O H H O HCl OH H H 2.) In the case of a Mannich reaction, the electrophile (an imine) must also be made. Draw the mechanism for the forward and backward reaction: O MeNH2 N H H cat. AcOH H H 3.) When a secondary amine is used and the carbonyl has an enolizable proton, an enamine can be formed. Are enamines nucleophilic or electrophilic? Nucleophilic H O N N H2O H cat. AcOH H enamine N N H H enamine 4.) The following reaction is the Lobry-de-Bruyn-von-Ekenstein reaction. Don’t worry about the name. I only recently found out that that was the official name for the rearrangement leading from glucose to fructose or mannose and back again. I was taught it under a different name: the Carbohydrate Game. Under acidic or basic conditions, it is possible that the following sugars (glucose, fructose, and mannose) are in equilibrium with each other. Draw the acid catalyzed version and the base catalyzed version. (Hint: this is just a repeat of question 1.) O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose The sugars are drawn in a Fisher projection (or what I think of is a dead cat projection). -

Heterocyclic Chemistrychemistry
HeterocyclicHeterocyclic ChemistryChemistry Professor J. Stephen Clark Room C4-04 Email: [email protected] 2011 –2012 1 http://www.chem.gla.ac.uk/staff/stephenc/UndergraduateTeaching.html Recommended Reading • Heterocyclic Chemistry – J. A. Joule, K. Mills and G. F. Smith • Heterocyclic Chemistry (Oxford Primer Series) – T. Gilchrist • Aromatic Heterocyclic Chemistry – D. T. Davies 2 Course Summary Introduction • Definition of terms and classification of heterocycles • Functional group chemistry: imines, enamines, acetals, enols, and sulfur-containing groups Intermediates used for the construction of aromatic heterocycles • Synthesis of aromatic heterocycles • Carbon–heteroatom bond formation and choice of oxidation state • Examples of commonly used strategies for heterocycle synthesis Pyridines • General properties, electronic structure • Synthesis of pyridines • Electrophilic substitution of pyridines • Nucleophilic substitution of pyridines • Metallation of pyridines Pyridine derivatives • Structure and reactivity of oxy-pyridines, alkyl pyridines, pyridinium salts, and pyridine N-oxides Quinolines and isoquinolines • General properties and reactivity compared to pyridine • Electrophilic and nucleophilic substitution quinolines and isoquinolines 3 • General methods used for the synthesis of quinolines and isoquinolines Course Summary (cont) Five-membered aromatic heterocycles • General properties, structure and reactivity of pyrroles, furans and thiophenes • Methods and strategies for the synthesis of five-membered heteroaromatics