Mechanism of Calcium Transport Stimulated by Chlorothiazide in Mouse Distal Convoluted Tubule Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -
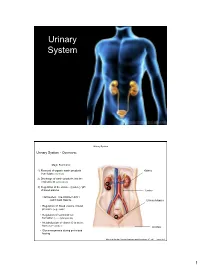
Urinary System
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -

Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion of Extracellular Fluid
Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion of Extracellular Fluid John P. Hayslett, … , Michael Kashgarian, Franklin H. Epstein J Clin Invest. 1967;46(7):1254-1263. https://doi.org/10.1172/JCI105618. Research Article Acute infusions of isotonic saline in the rat cause an increase in glomerular filtration rate and in the excretion of salt and water. The kidney swells, due to expansion of tubular and interstitial volume. Despite the increase in tubular diameter, transit time through the proximal tubules and loops of Henle is decreased, presumably owing to a greatly accelerated rate of tubular flow. Proximal tubular reabsorption, measured in blocked tubules, is inhibited in a way that cannot be ascribed to changes in tubular diameter. The prolongation of proximal reabsorptive half-time is not affected by the administration of aldosterone. It occurs equally in rats chronically loaded with or deprived of salt, and it is therefore not likely that it is influenced by the renal content of renin. In contrast, reabsorption from the distal convoluted tubule is enhanced by saline infusion. This change is observed in segments of tubules blocked with oil and isolated from their glomeruli and thus appears to occur independently of changes in glomerular filtration or tubular flow. Find the latest version: https://jci.me/105618/pdf Journal of Clinical Investigation Vol. 46, No. 7, 1967 Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion 'of Extracellular Fluid * JOHN P. HAYSLETT,t MICHAEL KASHGARIAN,4 AND FRANKLIN H. EPSTEIN § (From the Departments of Internal Medicine and Pathology, Yale University School of Medicine, New Haven, Conn.) Summary. -
Osmolality of Distal Tubular Fluid in the Dog
Osmolality of distal tubular fluid in the dog. J R Clapp, R R Robinson J Clin Invest. 1966;45(12):1847-1853. https://doi.org/10.1172/JCI105488. Research Article Find the latest version: https://jci.me/105488/pdf Journal of Clinical Investigation Vol. 45, No. 12, 1966 Osmolality of Distal Tubular Fluid in the Dog * JAMES R. CLAPP t AND ROSCOE R. ROBINSON t (From the Department of Medicine, Duke University Medical Center, Durham, N. C.) Renal micropuncture observations in rodents suggested that dog distal fluid is normally hypo- have demonstrated that the tubular fluid from early tonic rather than isotonic at the end of the distal portions of the distal convoluted tubule is always convolution. If so, at high rates of urine flow dur- hypotonic to plasma (1, 2). A limited number of ing osmotic diuresis, the ability of the collecting observations have suggested that its hypotonicity ducts to abstract solute-free water might be so is maintained along the entire distal tubule dur- exceeded that hypotonic urine could be excreted ing water diuresis (1). In contrast, during hy- despite the presence of antidiuretic hormone. dropenia with or without a superimposed osmotic Possible relationships among rates of solute excre- diuresis, the distal fluid achieves osmotic equi- tion, vasopressin dosage, and solute-free water librium with plasma in later portions of the distal reabsorption by the collecting ducts have been tubule (1, 2). Consequently, in the presence of discussed fully by Orloff, Wagner, and Davidson antidiuretic hormone, it has been widely accepted (11). that distal tubular fluid is always isosmotic to Heretofore, direct measurements of distal fluid plasma as it enters the cortical collecting ducts. -

3 PART KIDNEY MODEL Interlobular Artery & Vein
3 PART KIDNEY MODEL Interlobular Artery & Vein Renal Pelvis Arcuate Artery & Vein Calyces Interlobar Artery & Vein Renal Papilla Renal Artery Renal Pyramid Renal Vein Hilum Renal Column Opening of Kidney Area between renal pyramids Ureter Medulla Inner Region Renal Sinus Cavity beneath Cortex renal pelvis Outer Region Renal Capsule Whole Kidney 3 PART KIDNEY MODEL Nephron Bowman’s Capsule Distal Convoluted Tubule Glomerulus Interlobular Artery & Vein Arcuate Artery Proximal Convoluted Tubule & Vein Collecting Duct Interlobar Vein & Artery Descending Limb of Loop of Henle Ascending Limb of Loop of Henle Loop of Henle 3 PART KIDNEY MODEL Corpuscle Afferent Arteriole Efferent Arteriole Juxtaglomerular Apparatus Glomerulus Visceral Layer Of the bowman’s Bowman’s Capsule capsule made up of Podocytes Parietal Layer Of the bowman’s capsule 3 PART KIDNEY MODEL Whole Kidney 3 PART KIDNEY MODEL Nephron 3 PART KIDNEY MODEL Corpuscle Of the bowman’s capsule made up of _________________ Of the bowman’s capsule MALE REPRODUCTIVE MODELS Ductus Inguinal Canal Deferens Urinary Urinary Bladder Bladder Ductus Seminal Deferens Vesicle Seminal Prostate Gland Vesicle Prostate Inguinal Gland Canal Spermatic Spermatic With Cord With Cord Testicular Testicular Artery& Artery& Vein Vein Penis Penis Epididymis Epididymis Glans Testes Glans Testes Scrotum Scrotum MALE REPRODUCTIVE MODELS Urinary Urinary Bladder Bladder Spongy Ejaculatory Urethra DuctCC Prostatic Prostatic Urethra Urethra Prostate Prostate Gland Gland Ejaculatory Corpora Duct Cavernosa Corpora Corpus -

Histology of Urinary System
HISTOLOGY OF URINARY SYSTEM Dr. Rajesh Ranjan Assistant Professor Deptt. of Veterinary Anatomy C.V.Sc. & A.H., Rewa The main organs of this system are: Kidneys Ureters Urinary bladder Urethra KIDNEY Comprises of Capsule Parenchyma Cortex Medulla Cortical labyrinth Medullary rays Outer Medulla Inner Medulla Outer stripes Inner stripes Capsule is made up of collagen fibers, some smooth muscle fibers and blood capillaries. The Parenchyma consists of millions of nephrons, branches of renal arteries, veins, lymphatics and nerves. The Nephrons are the structural and functional unit of kidney. Nephrons can be classified On the basis of location of their glomeruli: Superficial (near the capsule) Mid cortical (near the medulla/Juxtamedullary) On the basis of the length of the loop of henle: Short looped- generally have superficial or mid cortical glomeruli and the tubules extend only into the outer medulla before it reflects back into the cortex. Long looped- have juxtamedullary glomeruli and tubules extend into the inner medulla before reflecting back into the cortex. Nephrons comprises of: 1. Renal corpuscles Glomerulus Glomerular capillaries Mesangiam Bowman’s capsule Parietal layer Visceral layer 2. Proximal tubules Proximal convoluted tubule (PCT) Proximal straight tubule (PST) 3. Henle’s loop Thin descending portion Thin ascending portion Thick ascending portion 4. Distal convoluted tubule 5. Connecting segment 6. Collecting duct Arcade- initial collecting tubule Straight portion- Cortical collecting duct Outer medullary -

The Urinary System Consists of Kidneys, Ureters, Urinary Bladder
Anatomy Lecture Notes Chapter 23 the urinary system consists of kidneys, ureters, urinary bladder, and urethra the function of the kidneys is NOT "to make urine" the kidneys: 1) regulate water balance 2) regular ECF electrolyte levels (Na, K, Ca) 3) eliminate some metabolic wastes urine is a by-product of these functions A. kidneys 1. located against posterior abdominal wall (retroperitoneal) T11 or T12 to L3 right kidney lower than left kidney 2. surrounded by a. pararenal fat (posterior only) b. renal fascia c. adipose capsule - perirenal fat d. renal capsule - dense c.t. covering surface of kidney e. parietal peritoneum Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 3. layers a. cortex - contains renal corpuscles and extends inwards as renal columns b. medulla - consists of renal pyramids which consist mostly of collecting ducts papilla - apex of renal pyramid; where collecting ducts drain into calyx 4. cavities and associated structures a. renal sinus - space in medial part of kidney; contains renal pelvis b. renal pelvis - expanded superior part of ureter minor calyx collects urine from one renal papilla major calyx formed by junction of 2 or more minor calyces renal pelvis formed by junction of all major calyces Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 5. renal hilum - medial indentation; where ureter leaves kidney 6. blood flow through the kidney - renal fraction = 20% of cardiac output aorta renal artery segmental arteries lobar arteries interlobar arteries arcuate arteries cortical radiate (interlobular) arteries afferent arterioles glomerular capillaries (glomerulus) efferent arteriole peritubular capillaries and vasa recta cortical radiate (interlobular) veins arcuate veins interlobar veins renal vein inferior vena cava Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 7. -

Renal-Tubule Epithelial Cell Nomenclature for Single-Cell RNA-Sequencing Studies
REVIEW www.jasn.org Renal-Tubule Epithelial Cell Nomenclature for Single-Cell RNA-Sequencing Studies Lihe Chen,1 Jevin Z. Clark,1 Jonathan W. Nelson,2 Brigitte Kaissling,3 David H. Ellison ,2 and Mark A. Knepper1 1Epithelial Systems Biology Laboratory, Systems Biology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland; 2Division of Nephrology and Hypertension, Oregon Health & Science University, Portland, Oregon; and 3Institute of Anatomy, University of Zurich, Zurich, Switzerland The assignment of cell type names to also include information on the per- the International Union of Physiologic individual cell clusters in single-cell centage of each epithelial cell type in Sciences and published in in Kidney RNA-sequencing (RNA-seq; including mouse kidney and on widely accepted International in 1988.3 The 1988 nomen- single-nucleus RNA-seq) studies is a mRNA/protein markers that are consid- clature was focused on what to call each critical bioinformatic step because re- ered prerequisites for identification of renal tubule segment, and not neces- searchers will need to be able to reliably individual cell types. sarily what to call individual cell types. map the pertinent transcriptomes to The mammalian renal tubule is made Accordingly, we refocused the recom- other types of information in the litera- up of at least 14 segments, containing at mended terminology to the individual ture. Ambiguous or redundant cell-type least 16 distinct epithelial cell types.4–6 cell types present in the various -
HISTOLOGY of URINARY ORGANS 1. Kidney
LECTURE 4b URINARY SYSTEM (DR. B.M. KAVOI) • Main components of this system- 1. Kidneys, 2. Ureter, 3. Urinary bladder and 4. Urethra 1. KIDNEY • Parenchyma organized into cortex (F) and medulla (E) • Within parenchyma occur nephrones, collecting ducts, blood vessels, lymphatics and nerves • Parenchyma organized into lobes and lobules Lobes and lobules • Lobes are located btw adjacent renal columns with peripheral limits within medulla being the interlobar arteries • A renal lobule is defined as a portion of the kidney containing those nephrons that are drained by a common collecting duct. • At the cortex, the collecting duct lies at the axis of lobule, being surrounded by corticolabyrinth or network comprising of renal corpuscles, PCT and DCT • Lobules are centered on "medullary rays“, which are bundles of straight tubules (collecting ducts and loops of Henle) • Within the cortex, peripheral limits of a lobule are the interlobular blood vessels while in medulla, limits of lobules are not defined Nephron • Tubules in which urine is formed (functional unit of the kidney • Form the most abundant tissue of renal parenchyma • Consist of 5 parts; i. Renal corposule, ii. Proximal convoluted tubule iii. Medullary loop (loop of Henle) iv. Distal convoluted tubule v. Collecting duct i. Renal corpuscle • Produces glomerular ultrafiltrate • Is a spherical structure comprising of a) cluster of blood vessels= glomerulus b) double walled envelope= glomerular or Bowman’s capsule • Efferent arterioles enter while the afferent arterioles leave the glomerulus -

Aandp2ch23lecture.Pdf
Chapter 23 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Urinary system rids the body of waste products • Kidneys also play important roles in blood volume, pressure, and composition • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filter blood plasma, excrete toxic wastes • Regulate blood volume, pressure, and osmolarity • Regulate electrolytes and acid-base balance • Secrete erythropoietin, which stimulates the production of red blood cells • Help regulate calcium levels by participating in calcitriol synthesis • Clear hormones from blood • Detoxify free radicals • In starvation, they synthesize glucose from amino acids 23-5 Retroperitoneal Position of the Kidney Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. -

The Urinary System: Part A
10/20/2014 PowerPoint® Lecture Slides Kidney Functions prepared by Barbara Heard, Atlantic Cape Community • Regulating total water volume and total College solute concentration in water • Regulating ECF ion concentrations C H A P T E R 25 • Ensuring long-term acid-base balance • Removal of metabolic wastes, toxins, drugs The Urinary System: Part A © Annie Leibovitz/Contact Press Images © 2013 Pearson Education, Inc. © 2013 Pearson Education, Inc. Kidney Functions Urinary System Organs • Endocrine functions • Kidneys - major excretory organs – Renin - regulation of blood pressure • Ureters - transport urine from kidneys to – Erythropoietin - regulation of RBC urinary bladder production • Urinary bladder - temporary storage • Activation of vitamin D reservoir for urine • Gluconeogenesis during prolonged fasting • Urethra transports urine out of body © 2013 Pearson Education, Inc. © 2013 Pearson Education, Inc. Figure 25.1 The urinary system. Figure 25.2b Position of the kidneys against the posterior body wall. Hepatic veins (cut) Esophagus (cut) Inferior vena cava Renal artery Adrenal gland Renal hilum Aorta Renal vein Kidney Iliac crest Ureter Rectum (cut) Uterus (part of female reproductive system) 12th rib Urinary bladder Urethra © 2013 Pearson Education, Inc. © 2013 Pearson Education, Inc. 1 10/20/2014 Internal Anatomy Homeostatic Imbalance • Renal cortex • Pyelitis – Granular-appearing superficial region – Infection of renal pelvis and calyces • Renal medulla • Pyelonephritis – Composed of cone-shaped medullary (renal) – Infection/inflammation of entire kidney pyramids • Normally - successfully treated with – Pyramids separated by renal columns antibiotics • Inward extensions of cortical tissue © 2013 Pearson Education, Inc. © 2013 Pearson Education, Inc. Figure 25.2a Position of the kidneys against the posterior body wall. Figure 25.3 Internal anatomy of the kidney.