Osmolality of Distal Tubular Fluid in the Dog
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calcium Phosphate Microcrystals in the Renal Tubular Fluid Accelerate Chronic Kidney Disease Progression
The Journal of Clinical Investigation RESEARCH ARTICLE Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression Kazuhiro Shiizaki,1,2 Asako Tsubouchi,3 Yutaka Miura,1 Kinya Seo,4 Takahiro Kuchimaru,5 Hirosaka Hayashi,1 Yoshitaka Iwazu,1,6,7 Marina Miura,1,6 Batpurev Battulga,8 Nobuhiko Ohno,8,9 Toru Hara,10 Rina Kunishige,3 Mamiko Masutani,11 Keita Negishi,12 Kazuomi Kario,12 Kazuhiko Kotani,7 Toshiyuki Yamada,7 Daisuke Nagata,6 Issei Komuro,13 Hiroshi Itoh,14 Hiroshi Kurosu,1 Masayuki Murata,3 and Makoto Kuro-o1 1Division of Anti-aging Medicine, Center for Molecular Medicine, Jichi Medical University, Shimotsuke, Japan. 2Yurina Medical Park, Shimotsuga, Japan. 3Graduate School of Arts and Sciences, University of Tokyo, Tokyo, Japan. 4Division of Cell and Molecular Medicine, 5Division of Cardiology and Metabolism, Center for Molecular Medicine, 6Division of Nephrology, Department of Internal Medicine, 7Department of Clinical Laboratory Medicine, and 8Division of Histology and Cell Biology, Department of Anatomy, Jichi Medical University, Shimotsuke, Japan. 9Division of Ultrastructural Research, National Institute for Physiological Sciences, Okazaki, Japan. 10Electron Microscopy Analysis Station, Research Network and Facility Service Division, National Institute for Materials Science, Tsukuba, Japan. 11Healthcare Business Unit, Nikon Corporation, Yokohama, Japan. 12Division of Cardiovascular Medicine, Department of Internal Medicine, Jichi Medical University, Shimotsuke, Japan. 13Department of Cardiovascular Medicine, Graduate School of Medicine, University of Tokyo, Tokyo, Japan. 14Division of Endocrinology, Metabolism and Nephrology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan. The Western pattern diet is rich not only in fat and calories but also in phosphate. -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

« Glomerulogenesis and Renal Tubular Differentiation : Role of Hnf1β »
THESE DE DOCTORAT DE L’UNIVERSITE PARIS DESCARTES Ecole doctorale « Bio Sorbonne Paris Cité », ED 562 Département Développement, Génétique, Reproduction, Neurobiologie et Vieillissement (DGRNV) Spécialité : Développement Présentée pour obtenir le titre de DOCTEUR de l’Université Paris Descartes « Glomerulogenesis and renal tubular differentiation : Role of HNF1 β » Par Mlle Arianna FIORENTINO Soutenance le 13 décembre 2016 Composition du jury : Mme. Evelyne Fischer Directrice de thèse M. Marco Pontoglio Examinateur M. Jean-Jacques Boffa Rapporteur M. Yves Allory Rapporteur M Rémi Salomon Examinateur M Jean-Claude Dussaule Examinateur Equipe "Expression Génique, Développement et Maladies" (EGDM) INSERM U1016/ CNRS UMR 8104 / Université Paris-Descartes Institut Cochin, Dpt. Développement, Reproduction et Cancer 24, Rue du Faubourg Saint Jacques, 75014 Paris, France A. Fiorentino HNF1beta in kidney development “Connaître ce n'est pas démontrer, ni expliquer. C'est accéder à la vision.” (Le Petit Prince- Antoine de Saint-Exupéry) 2 A. Fiorentino HNF1beta in kidney development Aknowledgments - Remerciements – Ringraziamenti During this long adventure of the PhD, I was surrounded by many people that I will try to thank to in these pages. In first place, I would like to thank the members of the jury that have kindly accepted to evaluate my work: Jean-Jacques Boffa, Yves Allory, Jean-Claude Dussaule and Rémi Salomon. For the supervision and the precious advices, I would like to thank Evelyne Fischer and Marco Pontoglio that overviewed all my work. I thank Evelyne, my thesis director, for the scientific exchanges of ideas, for the guidance to complete my project and for her help in difficult moments. I thank Marco for the discussions, even for the heated ones, because the pressure in the environment not only helped me to work harder on science but more importantly on my character, to face the problems and solve them. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -
Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink. -
![L8-Urine Conc. [PDF]](https://docslib.b-cdn.net/cover/4402/l8-urine-conc-pdf-1384402.webp)
L8-Urine Conc. [PDF]
The loop of Henle is referred to as countercurrent multiplier and vasa recta as countercurrent exchange systems in concentrating and diluting urine. Explain what happens to osmolarity of tubular fluid in the various segments of the loop of Henle when concentrated urine is being produced. Explain the factors that determine the ability of loop of Henle to make a concentrated medullary gradient. Differentiate between water diuresis and osmotic diuresis. Appreciate clinical correlates of diabetes mellitus and diabetes insipidus. Fluid intake The total body water Antidiuretic hormone is controled by : Renal excretion of water Hyperosmolar medullary Changes in the osmolarity of tubular fluid : interstitium 1 2 3 Low osmolarity The osmolarity High osmolarity because of active decrease as it goes up because of the transport of Na+ and because of the reabsorbation of water co-transport of K+ and reabsorption of NaCl Cl- 4 5 Low osmolarity because of High osmolarity because of reabsorption of NaCl , also reabsorption of water in reabsorption of water in present of ADH , present of ADH reabsorption of urea Mechanisms responsible for creation of hyperosmolar medulla: Active Co- Facilitated diffusion transport : transport : diffusion : of : Na+ ions out of the Only of small thick portion of the K+ , Cl- and other amounts of water ascending limb of ions out of the thick from the medullary the loop of henle portion of the Of urea from the tubules into the into the medullary ascending limb of inner medullary medullary interstitium the loop of henle collecting -
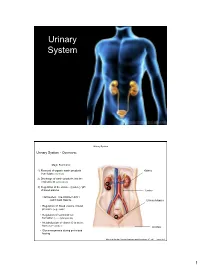
Urinary System
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -

A Study of the Effect of Protein in Tubular Fluid in Proximal Tubular Reabsorption Robert Lee Mitchell Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1964 A study of the effect of protein in tubular fluid in proximal tubular reabsorption Robert Lee Mitchell Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Mitchell, Robert Lee, "A study of the effect of protein in tubular fluid in proximal tubular reabsorption" (1964). Yale Medicine Thesis Digital Library. 2943. http://elischolar.library.yale.edu/ymtdl/2943 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. YALE UNIVERSITY LIBRARY 3 9002 06679 0909 A STUDY OF THE EFFECT OF PROTEIN IN TUBULAR FLUID IN OXIMAL TUBULAR REABSORPTION ROBERT L. MITCHELL 19 6 4 YALE MEDICAL LIBRARY Digitized by the Internet Archive in 2017 with funding from The National Endowment for the Humanities and the Arcadia Fund https://archive.org/details/studyofeffectofpOOmitc A STUDY OF THE EFFECT OF PROTEIN IN TUBULAR FLUID IN PROXIMAL TUBULAR REABSORPTION by Robert L, Mitchell (B.A. DePauw I960) A Thesis Presented to the Faculty of the Yale University School of Medicine in Partial Fulfillment of the Requirements for the Degree of Doctor of Medicine Yale University School of Medicine 196U ACKNOWLEDGMENT With sincere appreciation* I wish to express my gratitude to Dr. -

Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion of Extracellular Fluid
Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion of Extracellular Fluid John P. Hayslett, … , Michael Kashgarian, Franklin H. Epstein J Clin Invest. 1967;46(7):1254-1263. https://doi.org/10.1172/JCI105618. Research Article Acute infusions of isotonic saline in the rat cause an increase in glomerular filtration rate and in the excretion of salt and water. The kidney swells, due to expansion of tubular and interstitial volume. Despite the increase in tubular diameter, transit time through the proximal tubules and loops of Henle is decreased, presumably owing to a greatly accelerated rate of tubular flow. Proximal tubular reabsorption, measured in blocked tubules, is inhibited in a way that cannot be ascribed to changes in tubular diameter. The prolongation of proximal reabsorptive half-time is not affected by the administration of aldosterone. It occurs equally in rats chronically loaded with or deprived of salt, and it is therefore not likely that it is influenced by the renal content of renin. In contrast, reabsorption from the distal convoluted tubule is enhanced by saline infusion. This change is observed in segments of tubules blocked with oil and isolated from their glomeruli and thus appears to occur independently of changes in glomerular filtration or tubular flow. Find the latest version: https://jci.me/105618/pdf Journal of Clinical Investigation Vol. 46, No. 7, 1967 Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion 'of Extracellular Fluid * JOHN P. HAYSLETT,t MICHAEL KASHGARIAN,4 AND FRANKLIN H. EPSTEIN § (From the Departments of Internal Medicine and Pathology, Yale University School of Medicine, New Haven, Conn.) Summary. -

The Distal Convoluted Tubule and Collecting Duct
Chapter 23 *Lecture PowerPoint The Urinary System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Urinary system rids the body of waste products. • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filters blood plasma, separates waste from useful chemicals, returns useful substances to blood, eliminates wastes • Regulate blood volume and pressure by eliminating or conserving water • Regulate the osmolarity of the body fluids by controlling the relative amounts of water and solutes -

Filtration of Protein in the Anti-Glomerular Basement Membrane Nephritic Rat: a Micropuncture Study
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 10 (1976) p. 425—437 Filtration of protein in the anti-glomerular basement membrane nephritic rat: A micropuncture study HANS VON BAEYER, JUDITH B. VAN LIEW, JOHN KLASSEN and JOHN W. BOYLAN with the technical assistance of NANCY MANZ and PATRICIA MUIR Departments of Medicine, Physiology and Microbiology, State University of New York at Buffalo and Veterans Administration Hospital, Buffalo, New York Filtration of protein in the anti-glomerular basement membrane (GBM). We have used this animal model to examine, nephritic rat: A micropuncture study. Production on an anti-gb- with micropuncture techniques, changes in the filtra- merular basement membrane (anti-GBM) nephritis in the rat re- sults in a 30-fold increase in glomerular membrane permeability to tion and reabsorption of protein during the first to albumin. The concentration of albumin in glomerular filtrate, esti- third weeks of the disease (days 2 to 17) and to mated from proximal tubular fluid samples, is ten times the normal correlate some of the renal functional deficits ob- value. Tubular reabsorption of albumin is not enhanced so that essentially the filtered load is excreted. A nephrotic syndrome served with pathological findings. The period of develops rapidly. Total kidney gbomerular filtration rate (GFR) is study therefore complements the work of Baldamus reduced to 40% of normal with a proportional reduction in filtra- et al [1] on changes in protein excretion during the tion fraction. Glomerulo-tubular balance is maintained since prox- imal fractional reabsorption remains constant near control levels. -
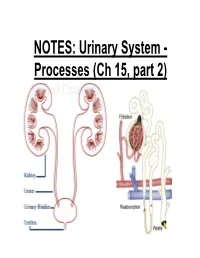
NOTES: Urinary System
NOTES: Urinary System - Processes (Ch 15, part 2) *Recall: the primary function of the urinary system is to filter the blood of ions and nitrogenous wastes; when combined with water, these wastes make up URINE. • Kidneys receive about 20-25% of total cardiac output – ~1200mL of blood goes through the kidneys per minute Blood Supply to Kidneys • Descending aorta Renal artery interlobar arcuate cortical afferent arterioles • The afferent arterioles deliver blood to nephrons NEPHRONS • NEPHRONS: the functional units of the kidneys -each kidney contains about a million nephrons! Parts of a NEPHRON: • GLOMERULUS: tangled cluster of blood capillaries • GLOMERULAR CAPSULE (a.k.a. Bowman’s capsule): thin-walled structure surrounding glomerulus Parts of a NEPHRON: • PROXIMAL CONVOLUTED TUBULE • LOOP OF HENLE -descending limb -ascending limb • DISTAL CONVOLUTED TUBULE Parts of a NEPHRON: • COLLECTING DUCT (where distal tubules from several nephrons converge and drain into; from here, urine empties into the RENAL PELVIS) Blood Supply of a Nephron: -blood is brought to a nephron from an afferent arteriole; -from here, it is passed to an efferent arteriole; -this gives rise to a system of peritubular capillaries that surround the renal tubules URINE FORMATION *nephrons remove wastes from blood and regulate water and electrolyte concentrations. URINE IS THE END PRODUCT! Three Organic Wastes Products of Urine 1) Urea: most abundant, from breakdown of amino acids 2) Creatinine: generated in skeletal muscle when creatine phosphate is broken down (creatine