Aandp2ch23lecture.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Female Urethra
OBJECTIVES: • By the end of this lecture, student should understand the anatomical structure of urinary system. General Information Waste products of metabolism are toxic (CO2, ammonia, etc.) Removal from tissues by blood and lymph Removal from blood by Respiratory system And Urinary system Functions of the Urinary System Elimination of waste products Nitrogenous wastes Toxins Drugs Functions of the Urinary System Regulate homeostasis Water balance Acid-base balance in the blood Electrolytes Blood pressure Organs of the Urinary system Kidneys Ureters Urinary bladder Urethra Kidneys Primary organs of the urinary system Located between the 12th thoracic and 3rd lumbar vertebrae. Right is usually lower due to liver. Held in place by connective tissue [renal fascia] and surrounded by thick layer of adipose [perirenal fat] Each kidney is approx. 3 cm thick, 6 cm wide and 12 cm long Regions of the Kidney Renal cortex: outer region Renal medulla: pyramids and columns Renal pelvis: collecting system Kidneys protected by three connective tissue layers Renal fascia -Attaches to abdominal wall Renal capsule: -Surrounds each kidney -Fibrous sac -Protects from trauma and infection Adipose capsule -Fat cushioning kidney Nephrons Each kidney contains over a million nephrons [functional structure] • Blood enters the nephron from a network that begins with the renal artery. • This artery branches into smaller and smaller vessels and enters each nephron as an afferent arteriole. • The afferent arteriole ends in a specialized capillary called the Glomerulus. • Each kidney has a glomerulus contained in Bowman’s Capsule. • Any cells that are too large to pass into the nephron are returned to the venous blood supply via the efferent arteriole. -

Development and Structure of the Excretory System the Nephron
Biology 224 Human Anatomy and Physiology II Week 7; Lecture 1; Monday Dr. Stuart S. Sumida Development and Structure of the Excretory System The Nephron DEVELOPMENT AND STRUCTURE OF EXCRETORY SYSTEM EXCRETORY SYSTEM REVIEW • Kidneys derived from INTERMEDIATE MESODERM. • Kidney starts out as a SEGMENTAL STRUCTURE. • Bladder, as part of embryonic gut tube: lining derived from endoderm. • Note, this is EXCRETION, NOT “ELIMINATION.” EARLY KIDNEY DEVELOPMENT • There is a segment of intermediate mesoderm for every body segment. • Earliest kidney appears in the cervical region of the body! (About week 3.) Called the PRONEPHROS. • Develop very close to the gonads. (They battle it out for the nearby ducts.) THE PRONEPHROS • The early anteriorly developed kidney parts. • Has NO EXCRETORY FUNCTION. • Functions to INDUCE DEVELOPMENT of middle segments of intermediate meosderm into the MESONEPHROS. THE MESONEPHROS • Some think it is the functioning embryonic kidney. Some think is has no excretory function. • WE DO KNOW that the duct that attaches to it (THE MESONEPHRIC DUCT) is very important in INDUCING DEVELOPMENT OF THE CAUDAL KIDNEY SEGMENTS (the METANEPHROS). Mesonephric Duct reaches all the way to end of gut tube (cloaca). We need to... • Attach the ducts to the hindend kidney (the metanephros). • Split the bladder away from the gut tube. After the mesonephric duct attaches to cloaca, the embryonic URETER grows from caudal to cranial to attach to mass of metanephric kidney. A septum, the URORECTAL SEPTUM, grows between the more dorsal part of the gut tube and the more ventral part that will become the bladder. Note that attached to the bladder are right and left ueters, the allantois (an extraembryonic membrane). -

The Contribution of Nasal Countercurrent Heat Exchange to Water Balance in the Northern Elephant Seal, Mirounga Angustirostris
J. exp. Biol. 113, 447-454 (1984) 447 Printed in Great Britain © The Company of Biologists Limited 1984 THE CONTRIBUTION OF NASAL COUNTERCURRENT HEAT EXCHANGE TO WATER BALANCE IN THE NORTHERN ELEPHANT SEAL, MIROUNGA ANGUSTIROSTRIS BY ANTHONY C. HUNTLEY, DANIEL P. COSTA Long Marine Laboratory, Center for Marine Studies, University of California, Santa Cruz, U.SA. AND ROBERT D. RUBIN Department of Life Sciences, Santa Rosa Junior College, Santa Rosa, California, U.SA Accepted 29 May 1984 SUMMARY 1. Elephant seals fast completely from food and water for 1-3 months during terrestrial breeding. Temporal countercurrent heat exchange in the nasal passage reduces expired air temperature (Te) below body temperature (Tb)- 2. At a mean ambient temperature of 13'7°C, Te is 20*9 °C. This results in the recovery of 71-5 % of the water added to inspired air. 3. The amount of cooling of the expired air (Tb~Te) and the percentage of water recovery varies inversely with ambient temperature. 4. Total nasal surface area available for heat and water exchange, located in the highly convoluted nasal turbinates, is estimated to be 720 cm2 in weaned pups and 3140 cm2 in an adult male. 5. Nasal temporal countercurrent heat exchange reduces total water loss sufficiently to allow maintenance of water balance using metabolic water production alone. INTRODUCTION Northern elephant seals, Mirounga angustirostis (Gill), are exceptional among pin- nipeds in the duration of their terrestrial breeding fast. During this time, they volun- tarily forgo both food and water while remaining active on the rookery. The length of these fasts varies with age, sex and social status. -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

35-40 Institute of Normal Anatomy
35 Lymphology 28 (1995) 35-40 Institute of Normal Anatomy (PP,AT), Institute of Histology and General Embryology (CM), and Department of Human Pathology (RS), University of Pavia, Italy ABSTRACT light and electron microscopy (2,3). To clarify vesical lymph drainage, we now examined the After endoscopic transurethral biopsies of fine structure of small lymphatic vessels (SL V) normal human urinary bladder, an extensive and their distribution in the normal human network of small initial lymphatic vessels was urinary bladder. Particular attention was depicted by means of light and electron directed to the structure and composition of microscopy. Using light microscopy, lymphatic the connective matrix that surrounds the vessels were seen in the mucosa and lymphatic vessels in the different regions of submucosa and formed a complex network in the bladder wall. the detrusor muscular coat. These lymphatics were characterized by an irregular and MATERIALS AND METHODS attenuated wall and increased in number and size from the superficial to the deeper region of Ten male subjects (40 to 60 years of age) the bladder. Ultrastructurally, the lymphatic who had symptoms of bladder outlet wall was characterized by endothelial cells obstruction were utilized for this investigation. joined together end-to-end or by complicated Two endoscopic transurethral biopsies from interdigitations. Often intercellular channels the lateral wall of the normal urinary bladder and gaps between two contiguous endothelial were performed under a constant bladder cells were present. A broad network of elastic distention after introduction of physiological and collagen fibers joined the lymphatic saline at 50 cm H20 constant pressure. The endothelial wall to the neighboring connective biopsy trocar was regulated to obtain vesical tissue. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Clinical and Functional Anatomy of the Urethral Sphincter
Review Article International Neurourology Journal Int Neurourol J 2012;16:102-106 http://dx.doi.org/10.5213/inj.2012.16.3.102 pISSN 2093-4777 · eISSN 2093-6931 INJ Clinical and Functional Anatomy of the Urethral Sphincter Junyang Jung, Hyo Kwang Ahn, Youngbuhm Huh Department of Anatomy, Kyung Hee University School of Medicine, Seoul, Korea Continence and micturition involve urethral closure. Especially, insufficient strength of the pelvic floor muscles including the urethral sphincter muscles causes urinary incontinence (UI). Thus, it is most important to understand the main mechanism causing UI and the relationship of UI with the urethral sphincter. Functionally and anatomically, the urethral sphincter is made up of the internal and the external sphincter. We highlight the basic and clinical anatomy of the internal and the external sphinc- ter and their clinical meaning. Understanding these relationships may provide a novel view in identifying the main mechanism causing UI and surgical techniques for UI. Keywords: Urethral sphincters; Pudendal nerve; Autonomic nervous system; Urinary incontinence; Urination INTRODUCTION tomical damage to the ligaments, facial support, and pelvic floor musculature, including the levator ani [8]. The pudendal nerve The urethral sphincter is crucial for the maintenance of urinary innervating the EUS is susceptible to injury during vaginal birth continence [1,2]. The urethral sphincter refers to one of the fol- because it travels between the sacrospinous and sacrotuberous lowing muscles [3]: 1) the internal urethral sphincter (IUS), ligaments [9]. In this article, we discuss the basic and clinical which consists of smooth muscle and is continuous with the anatomy of the urethral sphincter and the relationship between detrusor muscle and under involuntary control, and 2) the ex- the urethral sphincter and UI. -

Renal Corpuscle Renal System > Histology > Histology
Renal Corpuscle Renal System > Histology > Histology Key Points: • The renal corpuscles lie within the renal cortex; • They comprise the glomerular, aka, Bowman's capsule and capillaries The capsule is a double-layer sac of epithelium: — The outer parietal layer folds upon itself to form the visceral layer. — The inner visceral layer envelops the glomerular capillaries. • As blood passes through the glomerular capillaries, aka, glomerulus, specific components, including water and wastes, are filtered to create ultrafiltrate. • The filtration barrier, which determines ultrafiltrate composition, comprises glomerular capillary endothelia, a basement membrane, and the visceral layer of the glomerular capsule. • Nephron tubules modify the ultrafiltrate to form urine. Overview Diagram: • Tuft of glomerular capillaries; blood enters the capillaries via the afferent arteriole, and exits via efferent arteriole. • The visceral layer of the glomerular capsule envelops the capillaries, then folds outwards to become the parietal layer. • The capsular space lies between the parietal and visceral layers; this space fills with ultrafiltrate. • Vascular pole = where the arterioles pass through the capsule • Urinary pole = where the nephron tubule begins • Distal tubule passes by the afferent arteriole. Details of Capillary and Visceral Layer: • Fenestrated glomerular capillary; fenestrations are small openings, aka, pores, in the endothelium that confer permeability. • Thick basement membrane overlies capillaries • Visceral layer comprises podocytes: — Cell bodies — Cytoplasmic extensions, called primary processes, give rise to secondary foot processes, aka, pedicles. • The pedicles interdigitate to form filtration slits; molecules pass through these slits to form the ultrafiltrate in the 1 / 3 capsular space. • Subpodocyte space; healthy podocytes do not adhere to the basement membrane. Clinical Correlation: • Podocyte injury causes dramatic changes in shape, and, therefore, their ability to filter substances from the blood. -
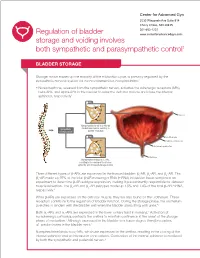
Regulation of Bladder Storage and Voiding Involves Both Sympathetic
Center for Advanced Gyn 5530 Wisconsin Ave Suite 914 Chevy Chase, MD 20815 301-652-1231 Regulation of bladder www.centerforadvancedgyn.com storage and voiding involves both sympathetic and parasympathetic control1 BLADDER STORAGE Storage, which makes up the majority of the micturition cycle, is primarily regulated by the sympathetic nervous system via the neurotransmitter, norepinephrine.2 • Norepinephrine, released from the sympathetic nerves, activates the adrenergic receptors (ARs), beta-ARs, and alpha-ARs in the bladder to relax the detrusor muscle and close the internal sphincter, respectively2 β-ARs Norepinephrine Ureter β-AR Norepinephrine binds to β-ARs on Sympathetic the detrusor muscle, resulting in nervous system bladder relaxation α-ARs Urothelium -AR α1 Detrusor muscle Internal sphincter Norepinephrine Urethra Norepinephrine binds to α1-ARs, resulting in the closing of the internal sphincter and increased storage of urine Three different types of b-ARs are expressed in the human bladder: b1-AR, b2-AR, and b3-AR. The b3-AR made up 97% of the total b-AR messenger RNA (mRNA) in bladder tissue samples in an experiment to determine b-AR subtype expression, making it predominantly responsible for detrusor muscle relaxation. The b1-AR and b2-AR subtypes made up 1.5% and 1.4% of the total b-AR mRNA, respectively.3 While b-ARs are expressed on the detrusor muscle, they are also found on the urothelium. These receptors contribute to the regulation of bladder function. During the storage phase, the urothelium stretches in tandem with the bladder wall when the bladder starts filling with urine.4,5 6 Both a1-ARs and a2-ARs are expressed in the lower urinary tract in humans. -

Kidney Function • Filtration • Reabsorption • Secretion • Excretion • Micturition
About This Chapter • Functions of the kidneys • Anatomy of the urinary system • Overview of kidney function • Filtration • Reabsorption • Secretion • Excretion • Micturition © 2016 Pearson Education, Inc. Functions of the Kidneys • Regulation of extracellular fluid volume and blood pressure • Regulation of osmolarity • Maintenance of ion balance • Homeostatic regulation of pH • Excretion of wastes • Production of hormones © 2016 Pearson Education, Inc. Anatomy of the Urinary System • Kidneys, ureters, bladder, and urethra • Kidneys – Bean-shaped organ – Cortex and medulla © 2016 Pearson Education, Inc. Anatomy of the Urinary System • Functional unit is the nephron – Glomerulus in the Bowman’s capsule – Proximal tubule – The loop of Henle • Descending limb and ascending limb twisted between arterioles forming the juxtaglomerular apparatus – Distal tubule – Collecting ducts © 2016 Pearson Education, Inc. Figure 19.1b Anatomy summary The kidneys are located retroperitoneally at the level of the lower ribs. Inferior Diaphragm vena cava Aorta Left adrenal gland Left kidney Right kidney Renal artery Renal vein Ureter Peritoneum Urinary Rectum (cut) bladder (cut) © 2016 Pearson Education, Inc. Figure 19.1c Anatomy summary © 2016 Pearson Education, Inc. Figure 19.1d Anatomy summary © 2016 Pearson Education, Inc. Figure 19.1f-h Anatomy summary Some nephrons dip deep into the medulla. One nephron has two arterioles and two sets of capillaries that form a portal system. Efferent arteriole Arterioles Peritubular Juxtaglomerular capillaries The cortex apparatus contains all Bowman’s Nephrons Afferent capsules, arteriole Glomerulus proximal Juxtamedullary nephron and distal (capillaries) with vasa recta tubules. Peritubular capillaries Glomerulus The medulla contains loops of Henle and Vasa recta collecting ducts. Collecting duct Loop of Henle © 2016 Pearson Education, Inc.