Differential Effects of Cisplatin in Proximal and Distal Renal Tubule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Control of Fluid Absorption in the Renal Proximal Tubule
Control of fluid absorption in the renal proximal tubule Maurice B. Burg, Jack Orloff J Clin Invest. 1968;47(9):2016-2024. https://doi.org/10.1172/JCI105888. Research Article Glomerulotubular balance was investigated in isolated, perfused rabbit proximal tubules in vitro in order to evaluate some of the mechanisms proposed to account for the proportionate relationship between glomerular filtration rate and fluid absorption generally observed in vivo. The rate of fluid transport from lumen to bath in proximal convoluted tubules in vitro was approximately equal to the estimated normal rate in vivo. The absorption rate in proximal straight tubules however was approximately one-half as great. If the mechanism responsible for maintenance of glomerulotubular balance is intrinsic to the proximal tubule, as has been proposed on the basis of micropuncture studies, the rate of fluid absorption in vitro should be directly related to the perfusion rate and/or tubule volume. In the present studies absorption rate was only minimally affected when perfusion rate was increased or the tubule distended. Thus, glomerulotubular balance is not mediated by changes in velocity of flow of the tubular fluid or tubular diameter and therefore is not an intrinsic property of the proximal tubule. It has also been proposed that glomerulotubular balance results from a humoral feedback mechanism in which angiotensin directly inhibits fluid absorption by the proximal convoluted tubule. In the present experiments, angiotensin was found to have no significant effect on absorption rate. Find the latest version: https://jci.me/105888/pdf Control of Fluid Absorption in the Renal Proximal Tubule MAURICE B. -
![L5 6 -Renal Reabsorbation and Secretation [PDF]](https://docslib.b-cdn.net/cover/2118/l5-6-renal-reabsorbation-and-secretation-pdf-252118.webp)
L5 6 -Renal Reabsorbation and Secretation [PDF]
Define tubular reabsorption, Identify and describe tubular secretion, Describe tubular secretion mechanism involved in transcellular and paracellular with PAH transport and K+ Glucose reabsorption transport. Identify and describe Identify and describe the Study glucose titration curve mechanisms of tubular characteristic of loop of in terms of renal threshold, transport & Henle, distal convoluted tubular transport maximum, Describe tubular reabsorption tubule and collecting ducts splay, excretion and filtration of sodium and water for reabsorption and secretion Identify the tubular site and Identify the site and describe Revise tubule-glomerular describe how Amino Acids, the influence of aldosterone feedback and describe its HCO -, P0 - and Urea are on reabsorption of Na+ in the physiological importance 3 4 reabsorbed late distal tubules. Mind Map As the glomerular filtrate enters the renal tubules, it flows sequentially through the successive parts of the tubule: The proximal tubule → the loop of Henle(1) → the distal tubule(2) → the collecting tubule → finally ,the collecting duct, before it is excreted as urine. A long this course, some substances are selectively reabsorbed from the tubules back into the blood, whereas others are secreted from the blood into the tubular lumen. The urine represent the sum of three basic renal processes: glomerular filtration, tubular reabsorption, and tubular secretion: Urinary excretion = Glomerular Filtration – Tubular reabsorption + Tubular secretion Mechanisms of cellular transport in the nephron are: Active transport Pinocytosis\ Passive Transport Osmosis “Active transport can move a solute exocytosis against an electrochemical gradient and requires energy derived from metabolism” Water is always reabsorbed by a Simple diffusion passive (nonactive) (Additional reading) Primary active (without carrier physical mechanism Secondary active The proximal tubule, reabsorb protein) called osmosis , transport large molecules such as transport Cl, HCO3-, urea , which means water proteins by pinocytosis. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Novel Tubular–Glomerular Interplay in Diabetic Kidney Disease Mediated
Clinical and Experimental Nephrology https://doi.org/10.1007/s10157-019-01719-4 INVITED REVIEW ARTICLE Novel tubular–glomerular interplay in diabetic kidney disease mediated by sirtuin 1, nicotinamide mononucleotide, and nicotinamide adenine dinucleotide Oshima Award Address 2017 Kazuhiro Hasegawa1 Received: 6 December 2018 / Accepted: 15 February 2019 © The Author(s) 2019 Abstract Tubules interact with glomeruli, which are composed of podocytes, parietal epithelial cells, mesangial cells, and glomerular endothelial cells. Glomerular–tubular balance and tubuloglomerular feedback are the two components of the tubular–glo- merular interplay, which has been demonstrated to play roles in physiological renal function and in diabetic kidney disease (DKD), in which proteins leaking from glomeruli arrive at tubular regions, leading to further tubular injury caused by the accumulation of proteinuria-inducing reactive oxygens species and various cytokines. In the current review, we present our recent work identifying a novel tubular–glomerular interplay in DKD mediated by sirtuin 1 and nicotinamide mononucleotide. Keywords Sirtuin 1 · Tubuloglomerular feedback · Diabetic kidney disease · Nicotinamide mononucleotide Introduction The longevity gene sirtuin 1 In this review, we summarize our studies revealing the novel We have demonstrated the role of SIRT1 in kidneys, par- roles of sirtuin 1 (SIRT1) and nicotinamide mononucleo- ticularly in DKD. Figure 1 outlines the basic characteristics tide (NMN) in the tubular–glomerular interplay in diabetic of SIRT1, one of the seven isoforms of mammalian sirtuins, kidney disease (DKD). First, we overview the basic func- which are found in specific intracellular compartments. tions of SIRT1 and NMN and changes i1 and NMN during The first sirtuin that was discovered was Sir2 in yeast [1]. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Prime Mover and Key Therapeutic Target in Diabetic Kidney Disease
Diabetes Volume 66, April 2017 791 Richard E. Gilbert Proximal Tubulopathy: Prime Mover and Key Therapeutic Target in Diabetic Kidney Disease Diabetes 2017;66:791–800 | DOI: 10.2337/db16-0796 The current view of diabetic kidney disease, based on estimated glomerular filtration rate (eGFR) decline (2). In meticulously acquired ultrastructural morphometry and recognition of these findings, the term diabetic kidney the utility of measuring plasma creatinine and urinary al- disease rather than diabetic nephropathy is now commonly bumin, has been almost entirely focused on the glomer- used. On the background of recent advances in the role of ulus. While clearly of great importance, changes in the the proximal tubule as a prime mover in diabetic kidney PERSPECTIVES IN DIABETES glomerulus are not the major determinant of renal prog- pathology, this review highlights key recent developments. nosis in diabetes and may not be the primary event in the Published mostly in the general scientific and kidney- development of diabetic kidney disease either. Indeed, specific literature, these advances highlight the pivotal advances in biomarker discovery and a greater appreci- role this part of the nephron plays in the initiation, pro- ation of tubulointerstitial histopathology and the role of gression, staging, and therapeutic intervention in diabetic tubular hypoxia in the pathogenesis of chronic kidney kidney disease. From a pathogenetic perspective, as illus- disease have given us pause to reconsider the current trated in Fig. 1 and as elaborated on further in this review, “glomerulocentric” paradigm and focus attention on the proximal tubule that by virtue of the high energy require- tubular hypoxia as a consequence of increased energy de- ments and reliance on aerobic metabolism render it par- mands and reduced perfusion combine with nonhypoxia- ticularly susceptible to the derangements of the diabetic related forces to drive the development of tubular atrophy fi state. -
Urinary System
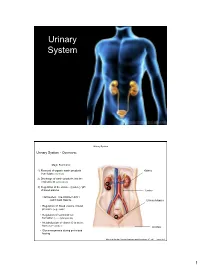
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -

(A) Adrenal Gland Inferior Vena Cava Iliac Crest Ureter Urinary Bladder
Hepatic veins (cut) Inferior vena cava Adrenal gland Renal artery Renal hilum Aorta Renal vein Kidney Iliac crest Ureter Rectum (cut) Uterus (part of female Urinary reproductive bladder system) Urethra (a) © 2018 Pearson Education, Inc. 1 12th rib (b) © 2018 Pearson Education, Inc. 2 Renal cortex Renal column Major calyx Minor calyx Renal pyramid (a) © 2018 Pearson Education, Inc. 3 Cortical radiate vein Cortical radiate artery Renal cortex Arcuate vein Arcuate artery Renal column Interlobar vein Interlobar artery Segmental arteries Renal vein Renal artery Minor calyx Renal pelvis Major calyx Renal Ureter pyramid Fibrous capsule (b) © 2018 Pearson Education, Inc. 4 Cortical nephron Fibrous capsule Renal cortex Collecting duct Renal medulla Renal Proximal Renal pelvis cortex convoluted tubule Glomerulus Juxtamedullary Ureter Distal convoluted tubule nephron Nephron loop Renal medulla (a) © 2018 Pearson Education, Inc. 5 Proximal convoluted Peritubular tubule (PCT) Glomerular capillaries capillaries Distal convoluted tubule Glomerular (DCT) (Bowman’s) capsule Efferent arteriole Afferent arteriole Cells of the juxtaglomerular apparatus Cortical radiate artery Arcuate artery Arcuate vein Cortical radiate vein Collecting duct Nephron loop (b) © 2018 Pearson Education, Inc. 6 Glomerular PCT capsular space Glomerular capillary covered by podocytes Efferent arteriole Afferent arteriole (c) © 2018 Pearson Education, Inc. 7 Filtration slits Podocyte cell body Foot processes (d) © 2018 Pearson Education, Inc. 8 Afferent arteriole Glomerular capillaries Efferent Cortical arteriole radiate artery Glomerular 1 capsule Three major renal processes: Rest of renal tubule 11 Glomerular filtration: Water and solutes containing smaller than proteins are forced through the filtrate capillary walls and pores of the glomerular capsule into the renal tubule. Peritubular 2 capillary 2 Tubular reabsorption: Water, glucose, amino acids, and needed ions are 3 transported out of the filtrate into the tubule cells and then enter the capillary blood. -

Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion of Extracellular Fluid
Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion of Extracellular Fluid John P. Hayslett, … , Michael Kashgarian, Franklin H. Epstein J Clin Invest. 1967;46(7):1254-1263. https://doi.org/10.1172/JCI105618. Research Article Acute infusions of isotonic saline in the rat cause an increase in glomerular filtration rate and in the excretion of salt and water. The kidney swells, due to expansion of tubular and interstitial volume. Despite the increase in tubular diameter, transit time through the proximal tubules and loops of Henle is decreased, presumably owing to a greatly accelerated rate of tubular flow. Proximal tubular reabsorption, measured in blocked tubules, is inhibited in a way that cannot be ascribed to changes in tubular diameter. The prolongation of proximal reabsorptive half-time is not affected by the administration of aldosterone. It occurs equally in rats chronically loaded with or deprived of salt, and it is therefore not likely that it is influenced by the renal content of renin. In contrast, reabsorption from the distal convoluted tubule is enhanced by saline infusion. This change is observed in segments of tubules blocked with oil and isolated from their glomeruli and thus appears to occur independently of changes in glomerular filtration or tubular flow. Find the latest version: https://jci.me/105618/pdf Journal of Clinical Investigation Vol. 46, No. 7, 1967 Changes in Proximal and Distal Tubular Reabsorption Produced by Rapid Expansion 'of Extracellular Fluid * JOHN P. HAYSLETT,t MICHAEL KASHGARIAN,4 AND FRANKLIN H. EPSTEIN § (From the Departments of Internal Medicine and Pathology, Yale University School of Medicine, New Haven, Conn.) Summary. -

Cell-Type Selective Markers Represented in Whole-Kidney RNA-Seq Data
bioRxiv preprint doi: https://doi.org/10.1101/348615; this version posted June 15, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Cell-Type Selective Markers Represented in Whole-Kidney RNA-Seq Data Jevin Z. Clark, B.S.*, Lihe Chen, PhD*, Chung-Lin Chou, PhD*, Hyun Jun Jung, PhD*, and Mark A. Knepper, MD, PhD* *Epithelial Systems Biology Laboratory, Systems Biology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland URunning titleU: RNA-Seq of Kidney UCorresponding AuthorU: Mark A. Knepper, MD, PhD 10 CENTER DR, MSC-1603 National Institutes of Health Bethesda, MD 20892-1603 Phone: 301-496-3064 Fax: 301-402-1443 Email: [email protected] Supplemental Datasets can be found at 33Thttps://hpcwebapps.cit.nih.gov/ESBL/Database/MouseWK/Data/ 33T 1 bioRxiv preprint doi: https://doi.org/10.1101/348615; this version posted June 15, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT Bulk-tissue RNA-Seq is seeing increasing use in the study of physiological and pathophysiological processes in the kidney. However, the presence of multiple cell types in kidney complicates the interpretation of the data. Here we address the question, “What cell types are represented in whole-kidney RNA-Seq data?” to identify circumstances in which bulk- kidney RNA-Seq can successfully be interpreted. We carried out RNA-Seq in mouse whole kidneys and microdissected proximal S2 segments.