5.2.9 Marteiliasis and Marteilioidiasis of Shellfish - 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -
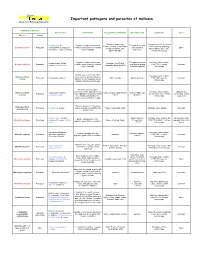
Pathogens Table 2006
Important pathogens and parasites of molluscs Genetic and Pathology Laboratory Pathogen or parasite Host species Host impact Geographical distribution Infection period Diagnostic Cycle Species Group Denmark, Netherland, Incubation period : 3-4 month Ostrea edulis, O. Parasite of oyster haemocytes Throughout the year France, Ireland, Great Britain in infected area. Histology, Bonamia ostreae Protozoan Conchaphila, O. Angasi, O. (=>all the tissues can be invaded). (with a peak in Direct (except Scotland), Italy, tissue imprint, PCR, ISH, Puelchana, Tiostrea chilensis Oyster mortality September) Spain and USA electron microscopy Parasite of oyster haemocytes Throughout the year Histology, tissue imprint, Ostrea angasi, Ostrea Australia, New Zéland, Bonamia exitiosa Protozoan (=>all the tissues can be invaded). (with a peak during PCR, ISH, electron Unknown chilensis, Ostrea edulis Tasmania, Spain (in 2007) Oyster mortality Australian autumn) microscopy Parasites present in connective Histology, tissue imprint, Haplosporidium tissue (mantle, gonads, digestive Protozoan Crassostrea virginica USA, Canada Spring-summer PCR, ISH, electron Unknown costale gland) but not in digestive tubule microscopy epithelia. Mortality in May-June Parasites present in gills, connective tissue. Sporulation only Histology, tissue imprint, Unknown but Haplosporidium Crassostrea virginica, USA, Canada, Japan, Korea, Summer (May until Protozoan in the epithelium of digestive gland smear, PCR, HIS, electron intermediate host is nelsoni Crassostrea gigas France October) in C. virginica, Mortalities in C. microscopy suspected virginica. No mortality in C. gigas Parasite present in connective Haplosporidium Protozoan O. edulis, O. angasi tissue. Sometimes, mortality can France, Netherland, Spain Histology, tissue imprint Unknown armoricanum be observed Ostrea edulis , Tiostrea Spring-summer Histology, tissue imprint, ISH, Intermediate host: Extracellular parasite of the Marteilia refringens Protozoan chilensis, O. -

Structure of Protistan Parasites Found in Bivalve Molluscs
W&M ScholarWorks VIMS Books and Book Chapters Virginia Institute of Marine Science 1988 Structure of Protistan Parasites Found in Bivalve Molluscs Frank O. Perkins Follow this and additional works at: https://scholarworks.wm.edu/vimsbooks Part of the Marine Biology Commons, and the Parasitology Commons American Fisheries Society Special Publication 18:93- 111 , 1988 CC> Copyrighl by !he American Fisheries Sociely 1988 PARASITE MORPHOLOGY, STRATEGY, AND EVOLUTION Structure of Protistan Parasites Found in Bivalve Molluscs 1 FRANK 0. PERKINS Virginia In stitute of Marine Science. School of Marine Science, College of William and Mary Gloucester Point, Virginia 23062, USA Abstral'I.-The literature on the structure of protists parasitizing bivalve molluscs is reviewed, and previously unpubli shed observations of species of class Perkinsea, phylum Haplosporidia, and class Paramyxea are presented. Descriptions are given of the flagellar apparatus of Perkin.His marinus zoospores, the ultrastructure of Perkinsus sp. from the Baltic macoma Maconw balthica, and the development of haplosporosome-like bodies in Haplosporidium nelsoni. The possible origin of stem cells of Marreilia sydneyi from the inner two sporoplasms is discussed. New research efforts are suggested which could help elucidate the phylogenetic interrelationships and taxonomic positions of the various taxa and help in efforts to better understand life cycles of selected species. Studies of the structure of protistan parasites terization of the parasite species, to elucidation of found in bivalve moll uscs have been fruitful to the many parasite life cycles, and to knowledge of morphologist interested in comparative morphol- parasite metabolism. The latter, especially, is ogy, evolu tion, and taxonomy. -

Shellfish Reefs at Risk
SHELLFISH REEFS AT RISK A Global Analysis of Problems and Solutions Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang CONTENTS Acknowledgments ........................................................................................................................ 1 Executive Summary .................................................................................................................... 2 Introduction .................................................................................................................................. 6 Methods .................................................................................................................................... 10 Results ........................................................................................................................................ 14 Condition of Oyster Reefs Globally Across Bays and Ecoregions ............ 14 Regional Summaries of the Condition of Shellfish Reefs ............................ 15 Overview of Threats and Causes of Decline ................................................................ 28 Recommendations for Conservation, Restoration and Management ................ 30 Conclusions ............................................................................................................................ 36 References ............................................................................................................................. -

Olympia Oyster (Ostrea Lurida)
COSEWIC Assessment and Status Report on the Olympia Oyster Ostrea lurida in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olympia Oyster Ostrea lurida in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 56 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 30 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Gillespie, G.E. 2000. COSEWIC status report on the Olympia Oyster Ostrea conchaphila in Canada in COSEWIC assessment and update status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-30 pp. Production note: COSEWIC acknowledges Graham E. Gillespie for writing the provisional status report on the Olympia Oyster, Ostrea lurida, prepared under contract with Environment Canada and Fisheries and Oceans Canada. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status reports were overseen by Robert Forsyth and Dr. Gerald Mackie, COSEWIC Molluscs Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l’huître plate du Pacifique (Ostrea lurida) au Canada. -

Aquaculture in Western Australia
Fact Sheet: Aquaculture in Western Australia Region North Coast, Gascoyne Coast, West Coast, South Coast Summary Despite Western Australia’s long coastline, our aquaculture industry is small by global standards – but it is growing and diversifying, with exciting opportunities on the horizon. Aside from contributing to food security, aquaculture creates employment and business opportunities in areas such as feed and equipment manufacturing. It also has direct and indirect economic benefits to the state, particularly in regional areas. In 2017-18, the total value of WA’s commercial fisheries and aquaculture production was $633 million. Of this, pearling (which is mostly commercially farmed) contributed $52 million (8%) and aquaculture $27 million (4%). Location of main aquaculture species farmed in Western Australia. Generated on 28/09/2021 https://marinewaters.fish.wa.gov.au/resource/fact-sheet-aquaculture-in-western-australia/ Page 1 of 8 Production for the Western Australian aquaculture industry in 2016/17 Current production – main aquaculture species in WA Barramundi Lates cacarifer Popular native table fish with high market demand which is sold as whole fish, live fish and fillets. WA’s barramundi production is largely located in the Kimberley region, at Cone Bay. Barramundi can be farmed in indoor recirculating systems, land-based ponds and sea cages. For more detailed information about barramundi aquaculture in Australia, see: https://www.agrifutures.com.au/farm-diversity/barramundi-aquaculture/ Barramundi farm in Cone Bay Rainbow trout Oncorhynchus mykiss Good eating fish that is popular with freshwater anglers. Trout was introduced to Australia for recreational fishing and aquaculture. It is difficult for the species to spawn naturally in WA’s conditions, so they are artificially bred in earthen and concrete ponds at the Pemberton Freshwater Research Centre. -

Ž Culture Potential of the Pearl Oyster Pinctada / Imbricata from The
Aquaculture 189Ž. 2000 375±388 www.elsevier.nlrlocateraqua-online Culture potential of the pearl oyster žPinctada imbricata/ from the Caribbean. II. Spat collection, and growth and mortality in culture systems H.-JorgÈ Urban) Facultad de Ciencias, Departamento de Biologõa, Seccion de Biologõa  Marina, UniÕersidad del Valle, A.A. 25360, Cali, Colombia Received 26 August 1999; received in revised form 23 February 2000; accepted 4 April 2000 Abstract Temporal variation in abundance of larvae and spat of the pearl oyster Pinctada imbricata was studied at several locations on the Colombian coast from May 1997 to June 1998. Larvae were sampled with bongo nets and spat were harvested from collectors at monthly intervals. Abun- dances of predators Ž.Cymatium gastropods, and portunid, xanthid and majiid crabs were also recorded. A relationship between salinity, particulate organic matter and larvae abundance was observed, leading to peaks in abundance of spat on collectors some weeks later. Average catch rates of 10 spat collectory1 monthy1, using collectors made of cheap easily accessible materials, indicate that availability of P. imbricata is sufficient to initiate and support aquaculture of this species. Growth and mortality rates of juveniles in three different culture systems at two densities Ž.20% and 30%, i.e. percentage of available area covered by juveniles showed that density within the same culture system had no effect on growth, but that growth differed significantly among the three culture systems. Growth in ``bag'' systems was lower than in boxes whereas growth in ``suspended'' and ``bottom'' boxes was similar and comparable to the growth of a natural population. -

NATURE TERRITORY April 2019 Newsletter of the Northern Territory Field Naturalists' Club Inc
NATURE TERRITORY April 2019 Newsletter of the Northern Territory Field Naturalists' Club Inc. In This Issue April Meeting p. 2 April Field Trip p. 3 Upcoming Activities p. 3 Death Adders pp. 4-5 Publications p. 5 Podcasts pp. 6-7 Chitter Chatter pp. 8-9 Club notices p. 10 Club web-site: http://ntfieldnaturalists.org.au/ Black-ringed Mangrove Snake (Hydrelaps darwiniensis) swimming around mangroves in Darwin Harbour. Photo: Nick Volpe FOR THE DIARY April Meeting: Wednesday 10 - Oysters going troppo ? research behind the recent success presented by Samantha Nowlands April Field Trip: Sunday 14 - Butterflies at East Point with Tissa Ratnayeke See pages 2 - 3 for m ore det ails Disclaimer: The views expressed in Nature Territory are not necessarily those of the NT Field Naturalists' Club Inc. or members of its Committee. April Meeting Oysters going troppo ? research behind the recent success presented by Samantha Nowland Wednesday 10, 7.45 pm, CDU Casuarina, Room BLUE 2.2.24 Sum m ary: Darwin Aquaculture Centre's (DAC) Black-lip Oyster hatchery research program and work with Aboriginal communities in South Goulburn Island has received a lot of media coverage in recent years, and it isn't stopping any time soon. DAC's collaboration with Traditional Owners in the Warruwi community on South Goulburn Island was showcased nationally this weekend on ABC's Landline program (https://www.abc.net.au/news/2019-03-30/native-oysters:-the-beginning-of-a-new-industry-in/10956306). The DAC team has worked extremely hard to get this program to where it is today. -

Is Pallial Mucus Involved in Ostrea Edulis Defenses Against the Parasite Bonamia
1 Is pallial mucus involved in Ostrea edulis defenses against the parasite Bonamia 2 ostreae? 3 Sergio Fernández-Boo1,4*, Ophélie Gervais1, Maria Prado-Alvarez3 Bruno Chollet1, 4 Stéphane Claverol2, Cyrielle Lecadet1, Christine Dubreuil1, Isabelle Arzul1 5 1 Institut Français de Recherche pour l´Exploitation de la Mer (IFREMER), Laboratoire de 6 Génétique et Pathologie (LGP), Avenue Mus de Loup, 17390 La Tremblade, France. 7 2 Université de Bordeaux, Centre Génomique Fonctionnelle de Bordeaux, Plateforme 8 Protéome, F-33000 Bordeaux, France. 9 3 Aquatic Molecular Pathobiology Group. Marine Research Institute (IIM-CSIC). Vigo. 10 Spain. 11 4 Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR), University of 12 Porto, Avenida General Norton de Matos, S/N, 4450-208 Matosinhos, Portugal. 13 14 *Corresponding author: [email protected] 15 Abstract 16 Bonamia ostreae is an intrahemocytic parasite that has been responsible for severe 17 mortalities in the flat oyster Ostrea edulis since the 1970´s. The Pacific oyster 18 Crassostrea gigas is considered to be resistant to the disease and appears to have 19 mechanisms to avoid infection. Most studies carried out on the invertebrate immune 20 system focus on the role of hemolymph, although mucus, which covers the body surface 21 of molluscs, could also act as a barrier against pathogens. In this study, the in vitro effect 22 of mucus from the oyster species Ostrea edulis and C. gigas on B. ostreae was 23 investigated using flow cytometry. Results showed an increase in esterase activities and 24 mortality rate of parasites exposed to mucus from both oyster species. -

Northern Australia Aquaculture Industry Situational Analysis Project A.1.1718119
Northern Australia Aquaculture Industry Situational Analysis Project A.1.1718119 Literature Review Editors: Jennifer Cobcroft and Dean Jerry Acknowledgments This research is funded by the CRC for Developing Northern Australia (CRCNA) is supported by the Cooperative Research Centres Program, an Australian Government initiative. The CRCNA also acknowledges the support of its investment partners: the Western Australian, Northern Territory and Queensland Governments. Disclaimer Any opinions expressed in this document are those of the authors. They do not purport to reflect the opinions or views of the CRCNA or its partners, agents or employees. The CRCNA gives no warranty or assurance and makes no representation as to the accuracy or reliability of any information or advice contained in this document, or that it is suitable for any intended use. The CRCNA, its partners, agents and employees, disclaim any and all liability for any errors or omissions or in respect of anything or the consequences of anything done or omitted to be done in reliance upon the whole or any part of this document. Peer Review Statement The CRCNA recognises the value of knowledge exchange and the importance of objective peer review. It is committed to encouraging and supporting its research teams in this regard. The author(s) confirm(s) that this document has been reviewed and approved by the project’s steering committee and by its program leader. These reviewers evaluated its: • originality • methodology • rigour • compliance with ethical guidelines • conclusions against results • conformity with the principles of the Australian Code for the Responsible Conduct of Research (NHMRC 2018), and provided constructive feedback which was considered and addressed by the author(s). -

Appendix L . Toxicity Assessment of Barossa
Appendix L . Toxicity Assessment of Barossa Condensate (Jacobs 2017) un-weathered Barossa Environmental Studies ConocoPhillips Toxicity Assessment of Barossa-3 Condensate IW021200-NMS-RP-0028 | Rev 1 30 May 2017 Toxicity Assessment of Barossa- 3 Condensate ConocoPhillips Toxicity Assessment of Barossa-3 Condensate Barossa Environmental Studies Project no: IW021200 Document title: Toxicity Assessment of Barossa-3 Condensate Document No.: IW021200-NMS-RP-0028 Revision: Rev 1 Date: 30 May 2017 Client name: ConocoPhillips Project manager: Chris Teasdale Author: Celeste Wilson File name: T:\Transfer\May2017\WVES\TMiley\Barossa Toxicity Report _ Rev 1.docx Jacobs Group (Australia) Pty Limited ABN 37 001 024 095 11th Floor, Durack Centre 263 Adelaide Terrace PO Box H615 Perth WA 6001 Australia T +61 8 9469 4400 F +61 8 9469 4488 www.jacobs.com © Copyright 2017 Jacobs Group (Australia) Pty Limited. The concepts and information contained in this document are the property of Jacobs. Use or copying of this document in whole or in part without the written permission of Jacobs constitutes an infringement of copyright. Limitation: This report has been prepared on behalf of, and for the exclusive use of Jacobs’ Client, and is subject to, and issued in accordance with, the provisions of the contract between Jacobs and the Client. Jacobs accepts no liability or responsibility whatsoever for, or in respect of, any use of, or reliance upon, this report by any third party. Document history and status Revision Date Description By Review Approved A 14/12/2015 Technical Review C Wilson M Huber C Teasdale 0 4/01/2016 Final report C Wilson C Teasdale C Teasdale 1 30/05/2017 Update report for inclusion in technical appendices of the C Wilson T Miley T Miley Barossa OPP IW021200-NMS-RP-0028 i Toxicity Assessment of Barossa-3 Condensate Contents Abbreviations and Glossary ................................................................................................................................ -

Feasibility of Shellfish Reef Restoration in a South-Western Australian Estuary
Feasibility of shellfish reef restoration in a south-western Australian estuary This thesis is presented for the degree of Bachelor of Science Honours, College of Science, Health, Engineering and Education, of Murdoch University, 2019 Lauren Peck (BSc) Declaration I declare that this thesis is my own account of my research and contains as its main content work which has not previously been submitted for a degree at any tertiary education institution. Lauren Peck Abstract With 85% of oyster reefs lost around the world within the last 130 years, these reefs are now one of the most threatened marine habitats in the world and in Australia less than 10% of naturally occurring oyster reefs remain. Shellfish reefs provide a range of services that promote healthy ecosystems, including water filtration, fish production and shoreline erosion. In estuaries, these services are extremely important as human activities are increasing degrading these environments. Thus, shellfish reefs can aid in restoring ecosystem functioning of an estuary while providing additional ecosystem services. The aim of this study was to determine the feasibility of a number of shellfish reef options for the Peel-Harvey Estuary in south-western Australia. This first involved exploring the historical and current distributions of shellfish to elucidate whether shellfish reefs existed in the Peel-Harvey Estuary and to identify a suite of candidate species. A bioclimatic modelling approach was then used to elucidate the suitability of five native Australian oyster species to the environmental conditions that occur in the Peel-Harvey Estuary, the largest estuary in south-western Australia. Laboratory tank trials were then used to validate the results of that model, in which the two most suitable species, i.e.