Other Diseases, Other Molluscs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -

Paleoenvironmental Interpretation of Late Glacial and Post
PALEOENVIRONMENTAL INTERPRETATION OF LATE GLACIAL AND POST- GLACIAL FOSSIL MARINE MOLLUSCS, EUREKA SOUND, CANADIAN ARCTIC ARCHIPELAGO A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Department of Geography University of Saskatchewan Saskatoon By Shanshan Cai © Copyright Shanshan Cai, April 2006. All rights reserved. i PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Head of the Department of Geography University of Saskatchewan Saskatoon, Saskatchewan S7N 5A5 i ABSTRACT A total of 5065 specimens (5018 valves of bivalve and 47 gastropod shells) have been identified and classified into 27 species from 55 samples collected from raised glaciomarine and estuarine sediments, and glacial tills. -

Performance of the New England Hydraulic Dredge for the Harvest Of'stimpson~Surf Clams
PERFORMANCE OF THE NEW ENGLAND HYDRAULIC DREDGE FOR THE HARVEST OF'STIMPSON~SURF CLAMS Inv d Experimental Biology Division*. Deparhnent of Fisheries and Oceans Canada Maurice Lamontagne Instihite 850 route de la Mer f O00 Mont-Joli (~uébec) G5H 324 Canadian Industry Report of isheries and Aquatic Sciences 235 ? nadian Industry Report of Fisheries and Aquatic Sciences canaain the ~e?iaIts:Bf ramh and develotrment iradustq fof MkF irnmedia~elar Iutuw1appdkashn. They are da.r.ected pfimarbly tmard indiziduak .h $ha primary and secondary çccrors of the fishing and marine mduaries. ?i,o ratlrMhn k phced on sulbjmt mcta and the series reflects the broad inoemrs and p~2ek5&oh Department d Fisher& and Oceans. aamely. fishiesad aqwticsc+awe~,. .' 1t@u~rr5reprts m. be cimed & full pnsblicatbns. The cornkt citatian aowars . abri the abatracr of each report. Eacb rekl is a&rrxad in Awair %ie.nr& ifid . technical publicatio . Sumber;l-9li e lndustrial Devel- opsnent BranCh. Technical Reports of th. lndiausrrkf ikxelo~mentÉranch. a& - ,Tecknical Reports of the &-sk*an's Servk Branch. kumkrs 62- I tO Giztaisseed as . Dapan~enzof Fishsies and the Em-i~onment.Fisher& and Màrine Service fndustry Reports. The current ysies name kas chmgel ~ithreport number 111. lndwsrr! reports are produc& rqiortdk bur are numkred nationrtlty. Requests .for indi\ idual rqporfs w il1 be fifkd by the issuing~rqblishmentl~tcdon the front cûvw and title page. Out-of-stwk feports-will k svpplied for fw? b'J; commacia~agents. Les rapporis 4 1'indlasrrk am t knmf k résultats des acîivitis de mher&c et de diveloppern-enl qui peuvent &se utiles a I'industrie gour des applications immédiates ou fatures. -

Circular 162. the Soft-Shell Clam
HE OFT iHELL 'LAM UNITED STATES DEPARTMENT 8F THE INTERIOR FISH AND WILDLIFE SERVICE BUREAU OF COMMERCIAL FISHERIES Circular 162 CONTENTS Page 1 Intr oduc tion. • • • . 2 Natural history • . ~ . 2 Distribution. 3 Taxonomy. · . Anatomy. • • • •• . · . 3 4 Life cycle. • • • • • . • •• Predators, diseases, and parasites . 7 Fishery - methods and management .•••.••• • 9 New England area. • • • • • . • • • • • . .. 9 Chesapeake area . ........... 1 1 Special problem s of paralytic shellfish poisoning and pollution . .............. 13 Summary . .... 14 Acknowledgment s •••• · . 14 Selected bibliography •••••• 15 ABSTRACT Describes the soft- shell cla.1n industry of the Atlantic coast; reviewing past and present economic importance, fishery methods, fishery management programs, and special problems associated with shellfish culture and marketing. Provides a summary of soft- shell clam natural history in- eluding distribution, taxonomy, anatomy, and life cycle. l THE SOFT -SHELL CLAM by Robert W. Hanks Bureau of Commercial Fisheries Biological Labor ator y U.S. Fish and Wildlife Serv i c e Boothbay Harbor, Maine INTRODUCTION for s a lted c l a m s used as bait by cod fishermen on t he G r and Bank. For the next The soft-shell clam, Myaarenaria L., has 2 5 years this was the most important outlet played an important role in the history and for cla ms a nd was a sour ce of wealth to economy of the eastern coast of our country . some coastal communities . Many of the Long before the first explorers reached digg ers e a rne d up t o $10 per day in this our shores, clams were important in the busin ess during October to March, when diet of some American Indian tribes. -
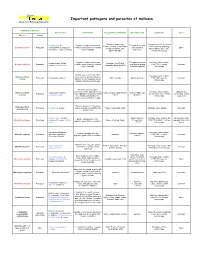
Pathogens Table 2006
Important pathogens and parasites of molluscs Genetic and Pathology Laboratory Pathogen or parasite Host species Host impact Geographical distribution Infection period Diagnostic Cycle Species Group Denmark, Netherland, Incubation period : 3-4 month Ostrea edulis, O. Parasite of oyster haemocytes Throughout the year France, Ireland, Great Britain in infected area. Histology, Bonamia ostreae Protozoan Conchaphila, O. Angasi, O. (=>all the tissues can be invaded). (with a peak in Direct (except Scotland), Italy, tissue imprint, PCR, ISH, Puelchana, Tiostrea chilensis Oyster mortality September) Spain and USA electron microscopy Parasite of oyster haemocytes Throughout the year Histology, tissue imprint, Ostrea angasi, Ostrea Australia, New Zéland, Bonamia exitiosa Protozoan (=>all the tissues can be invaded). (with a peak during PCR, ISH, electron Unknown chilensis, Ostrea edulis Tasmania, Spain (in 2007) Oyster mortality Australian autumn) microscopy Parasites present in connective Histology, tissue imprint, Haplosporidium tissue (mantle, gonads, digestive Protozoan Crassostrea virginica USA, Canada Spring-summer PCR, ISH, electron Unknown costale gland) but not in digestive tubule microscopy epithelia. Mortality in May-June Parasites present in gills, connective tissue. Sporulation only Histology, tissue imprint, Unknown but Haplosporidium Crassostrea virginica, USA, Canada, Japan, Korea, Summer (May until Protozoan in the epithelium of digestive gland smear, PCR, HIS, electron intermediate host is nelsoni Crassostrea gigas France October) in C. virginica, Mortalities in C. microscopy suspected virginica. No mortality in C. gigas Parasite present in connective Haplosporidium Protozoan O. edulis, O. angasi tissue. Sometimes, mortality can France, Netherland, Spain Histology, tissue imprint Unknown armoricanum be observed Ostrea edulis , Tiostrea Spring-summer Histology, tissue imprint, ISH, Intermediate host: Extracellular parasite of the Marteilia refringens Protozoan chilensis, O. -

The Impact of Hydraulic Blade Dredging on a Benthic Megafaunal Community in the Clyde Sea Area, Scotland
Journal of Sea Research 50 (2003) 45–56 www.elsevier.com/locate/seares The impact of hydraulic blade dredging on a benthic megafaunal community in the Clyde Sea area, Scotland C. Hauton*, R.J.A. Atkinson, P.G. Moore University Marine Biological Station Millport (UMBSM), Isle of Cumbrae, Scotland, KA28 0EG, UK Received 4 December 2002; accepted 13 February 2003 Abstract A study was made of the impacts on a benthic megafaunal community of a hydraulic blade dredge fishing for razor clams Ensis spp. within the Clyde Sea area. Damage caused to the target species and the discard collected by the dredge as well as the fauna dislodged by the dredge but left exposed at the surface of the seabed was quantified. The dredge contents and the dislodged fauna were dominated by the burrowing heart urchin Echinocardium cordatum, approximately 60–70% of which survived the fishing process intact. The next most dominant species, the target razor clam species Ensis siliqua and E. arcuatus as well as the common otter shell Lutraria lutraria, did not survive the fishing process as well as E. cordatum, with between 20 and 100% of individuals suffering severe damage in any one dredge haul. Additional experiments were conducted to quantify the reburial capacity of dredged fauna that was returned to the seabed as discard. Approximately 85% of razor clams retained the ability to rapidly rebury into both undredged and dredged sand, as did the majority of those heart urchins Echinocardium cordatum which did not suffer aerial exposure. Individual E. cordatum which were brought to surface in the dredge collecting cage were unable to successfully rebury within three hours of being returned to the seabed. -

Structure of Protistan Parasites Found in Bivalve Molluscs
W&M ScholarWorks VIMS Books and Book Chapters Virginia Institute of Marine Science 1988 Structure of Protistan Parasites Found in Bivalve Molluscs Frank O. Perkins Follow this and additional works at: https://scholarworks.wm.edu/vimsbooks Part of the Marine Biology Commons, and the Parasitology Commons American Fisheries Society Special Publication 18:93- 111 , 1988 CC> Copyrighl by !he American Fisheries Sociely 1988 PARASITE MORPHOLOGY, STRATEGY, AND EVOLUTION Structure of Protistan Parasites Found in Bivalve Molluscs 1 FRANK 0. PERKINS Virginia In stitute of Marine Science. School of Marine Science, College of William and Mary Gloucester Point, Virginia 23062, USA Abstral'I.-The literature on the structure of protists parasitizing bivalve molluscs is reviewed, and previously unpubli shed observations of species of class Perkinsea, phylum Haplosporidia, and class Paramyxea are presented. Descriptions are given of the flagellar apparatus of Perkin.His marinus zoospores, the ultrastructure of Perkinsus sp. from the Baltic macoma Maconw balthica, and the development of haplosporosome-like bodies in Haplosporidium nelsoni. The possible origin of stem cells of Marreilia sydneyi from the inner two sporoplasms is discussed. New research efforts are suggested which could help elucidate the phylogenetic interrelationships and taxonomic positions of the various taxa and help in efforts to better understand life cycles of selected species. Studies of the structure of protistan parasites terization of the parasite species, to elucidation of found in bivalve moll uscs have been fruitful to the many parasite life cycles, and to knowledge of morphologist interested in comparative morphol- parasite metabolism. The latter, especially, is ogy, evolu tion, and taxonomy. -

Shellfish Reefs at Risk
SHELLFISH REEFS AT RISK A Global Analysis of Problems and Solutions Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang CONTENTS Acknowledgments ........................................................................................................................ 1 Executive Summary .................................................................................................................... 2 Introduction .................................................................................................................................. 6 Methods .................................................................................................................................... 10 Results ........................................................................................................................................ 14 Condition of Oyster Reefs Globally Across Bays and Ecoregions ............ 14 Regional Summaries of the Condition of Shellfish Reefs ............................ 15 Overview of Threats and Causes of Decline ................................................................ 28 Recommendations for Conservation, Restoration and Management ................ 30 Conclusions ............................................................................................................................ 36 References ............................................................................................................................. -

Tracking Larval, Newly Settled, and Juvenile Red Abalone (Haliotis Rufescens ) Recruitment in Northern California
Journal of Shellfish Research, Vol. 35, No. 3, 601–609, 2016. TRACKING LARVAL, NEWLY SETTLED, AND JUVENILE RED ABALONE (HALIOTIS RUFESCENS ) RECRUITMENT IN NORTHERN CALIFORNIA LAURA ROGERS-BENNETT,1,2* RICHARD F. DONDANVILLE,1 CYNTHIA A. CATTON,2 CHRISTINA I. JUHASZ,2 TOYOMITSU HORII3 AND MASAMI HAMAGUCHI4 1Bodega Marine Laboratory, University of California Davis, PO Box 247, Bodega Bay, CA 94923; 2California Department of Fish and Wildlife, Bodega Bay, CA 94923; 3Stock Enhancement and Aquaculture Division, Tohoku National Fisheries Research Institute, FRA 3-27-5 Shinhamacho, Shiogama, Miyagi, 985-000, Japan; 4National Research Institute of Fisheries and Environment of Inland Sea, Fisheries Agency of Japan 2-17-5 Maruishi, Hatsukaichi, Hiroshima 739-0452, Japan ABSTRACT Recruitment is a central question in both ecology and fisheries biology. Little is known however about early life history stages, such as the larval and newly settled stages of marine invertebrates. No one has captured wild larval or newly settled red abalone (Haliotis rufescens) in California even though this species supports a recreational fishery. A sampling program has been developed to capture larval (290 mm), newly settled (290–2,000 mm), and juvenile (2–20 mm) red abalone in northern California from 2007 to 2015. Plankton nets were used to capture larval abalone using depth integrated tows in nearshore rocky habitats. Newly settled abalone were collected on cobbles covered in crustose coralline algae. Larval and newly settled abalone were identified to species using shell morphology confirmed with genetic techniques using polymerase chain reaction restriction fragment length polymorphism with two restriction enzymes. Artificial reefs were constructed of cinder blocks and sampled each year for the presence of juvenile red abalone. -

Xoimi AMERICAN COXCIIOLOGY
S31ITnS0NIAN MISCEllANEOUS COLLECTIOXS. BIBLIOGIIAPHY XOimi AMERICAN COXCIIOLOGY TREVIOUS TO THE YEAR 18G0. PREPARED FOR THE SMITHSONIAN INSTITUTION BY . W. G. BINNEY. PART II. FOKEIGN AUTHORS. WASHINGTON: SMITHSONIAN INSTITUTION. JUNE, 1864. : ADYERTISEMENT, The first part of the Bibliography of American Conchology, prepared for the Smithsonian Institution by Mr. Binuey, was published in March, 1863, and embraced the references to de- scriptions of shells by American authors. The second part of the same work is herewith presented to the public, and relates to species of North American shells referred to by European authors. In foreign works binomial authors alone have been quoted, and no species mentioned which is not referred to North America or some specified locality of it. The third part (in an advanced stage of preparation) will in- clude the General Index of Authors, the Index of Generic and Specific names, and a History of American Conchology, together with any additional references belonging to Part I and II, that may be met with. JOSEPH HENRY, Secretary S. I. Washington, June, 1864. (" ) PHILADELPHIA COLLINS, PRINTER. CO]^TENTS. Advertisement ii 4 PART II.—FOREIGN AUTHORS. Titles of Works and Articles published by Foreign Authors . 1 Appendix II to Part I, Section A 271 Appendix III to Part I, Section C 281 287 Appendix IV .......... • Index of Authors in Part II 295 Errata ' 306 (iii ) PART II. FOEEIGN AUTHORS. ( V ) BIBLIOGRxVPHY NOETH AMERICAN CONCHOLOGY. PART II. Pllipps.—A Voyage towards the North Pole, &c. : by CON- STANTiNE John Phipps. Loudou, ITTJc. Pa. BIBLIOGRAPHY OF [part II. FaliricillS.—Fauna Grcenlandica—systematice sistens ani- malia GrcEulandite occidentalis liactenus iudagata, &c., secun dum proprias observatioues Othonis Fabricii. -

Olympia Oyster (Ostrea Lurida)
COSEWIC Assessment and Status Report on the Olympia Oyster Ostrea lurida in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olympia Oyster Ostrea lurida in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 56 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 30 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Gillespie, G.E. 2000. COSEWIC status report on the Olympia Oyster Ostrea conchaphila in Canada in COSEWIC assessment and update status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-30 pp. Production note: COSEWIC acknowledges Graham E. Gillespie for writing the provisional status report on the Olympia Oyster, Ostrea lurida, prepared under contract with Environment Canada and Fisheries and Oceans Canada. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status reports were overseen by Robert Forsyth and Dr. Gerald Mackie, COSEWIC Molluscs Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l’huître plate du Pacifique (Ostrea lurida) au Canada. -

Enhancement of Red Abalone Haliotis Rufescens Stocks at San Miguel Island: Reassessing a Success Story
MARINE ECOLOGY PROGRESS SERIES Vol. 202: 303–308, 2000 Published August 28 Mar Ecol Prog Ser NOTE Enhancement of red abalone Haliotis rufescens stocks at San Miguel Island: reassessing a success story Ronald S. Burton1,*, Mia J. Tegner 2 1Marine Biology Research Division and 2 Marine Life Research Group, Scripps Institution of Oceanography, University of California, San Diego, La Jolla, California 92093-0202, USA ABSTRACT: Outplanting of hatchery-reared juvenile abalone quences of such practices? How can the success of has received much attention as a strategy for enhancement of costly outplants be assessed? Such questions point to depleted natural stocks. Most outplants attempted to date conflicting needs regarding the genetic composition of appear to have been unsuccessful. However, based on genetic analyses of a population sample taken in 1992, it has outplanted organisms. Where large numbers of organ- recently been suggested that a 1979 outplanting of red isms are artificially released into a depleted popula- abalone Haliotis rufescens, on the south side of San Miguel tion, substantial changes in the genetic composition of Island (California, USA), was successful and probably sus- natural populations may occur (e.g., Tringali & Bert tained the fishery there through the 1980s. We resampled the San Miguel population in 1999 and found no genetic signa- 1998, Utter 1998). A variety of scenarios suggest that ture of the outplants. Allelic frequencies in our 1999 sample wholesale changes in genetic composition of natural closely resemble those observed in a pre-outplant 1979 south- populations may be undesirable at best and potentially ern California sample and two 1999 northern California pop- disastrous.