Variance in the Reproductive Success of Flat Oyster Ostrea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -
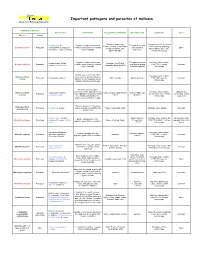
Pathogens Table 2006
Important pathogens and parasites of molluscs Genetic and Pathology Laboratory Pathogen or parasite Host species Host impact Geographical distribution Infection period Diagnostic Cycle Species Group Denmark, Netherland, Incubation period : 3-4 month Ostrea edulis, O. Parasite of oyster haemocytes Throughout the year France, Ireland, Great Britain in infected area. Histology, Bonamia ostreae Protozoan Conchaphila, O. Angasi, O. (=>all the tissues can be invaded). (with a peak in Direct (except Scotland), Italy, tissue imprint, PCR, ISH, Puelchana, Tiostrea chilensis Oyster mortality September) Spain and USA electron microscopy Parasite of oyster haemocytes Throughout the year Histology, tissue imprint, Ostrea angasi, Ostrea Australia, New Zéland, Bonamia exitiosa Protozoan (=>all the tissues can be invaded). (with a peak during PCR, ISH, electron Unknown chilensis, Ostrea edulis Tasmania, Spain (in 2007) Oyster mortality Australian autumn) microscopy Parasites present in connective Histology, tissue imprint, Haplosporidium tissue (mantle, gonads, digestive Protozoan Crassostrea virginica USA, Canada Spring-summer PCR, ISH, electron Unknown costale gland) but not in digestive tubule microscopy epithelia. Mortality in May-June Parasites present in gills, connective tissue. Sporulation only Histology, tissue imprint, Unknown but Haplosporidium Crassostrea virginica, USA, Canada, Japan, Korea, Summer (May until Protozoan in the epithelium of digestive gland smear, PCR, HIS, electron intermediate host is nelsoni Crassostrea gigas France October) in C. virginica, Mortalities in C. microscopy suspected virginica. No mortality in C. gigas Parasite present in connective Haplosporidium Protozoan O. edulis, O. angasi tissue. Sometimes, mortality can France, Netherland, Spain Histology, tissue imprint Unknown armoricanum be observed Ostrea edulis , Tiostrea Spring-summer Histology, tissue imprint, ISH, Intermediate host: Extracellular parasite of the Marteilia refringens Protozoan chilensis, O. -

Structure of Protistan Parasites Found in Bivalve Molluscs
W&M ScholarWorks VIMS Books and Book Chapters Virginia Institute of Marine Science 1988 Structure of Protistan Parasites Found in Bivalve Molluscs Frank O. Perkins Follow this and additional works at: https://scholarworks.wm.edu/vimsbooks Part of the Marine Biology Commons, and the Parasitology Commons American Fisheries Society Special Publication 18:93- 111 , 1988 CC> Copyrighl by !he American Fisheries Sociely 1988 PARASITE MORPHOLOGY, STRATEGY, AND EVOLUTION Structure of Protistan Parasites Found in Bivalve Molluscs 1 FRANK 0. PERKINS Virginia In stitute of Marine Science. School of Marine Science, College of William and Mary Gloucester Point, Virginia 23062, USA Abstral'I.-The literature on the structure of protists parasitizing bivalve molluscs is reviewed, and previously unpubli shed observations of species of class Perkinsea, phylum Haplosporidia, and class Paramyxea are presented. Descriptions are given of the flagellar apparatus of Perkin.His marinus zoospores, the ultrastructure of Perkinsus sp. from the Baltic macoma Maconw balthica, and the development of haplosporosome-like bodies in Haplosporidium nelsoni. The possible origin of stem cells of Marreilia sydneyi from the inner two sporoplasms is discussed. New research efforts are suggested which could help elucidate the phylogenetic interrelationships and taxonomic positions of the various taxa and help in efforts to better understand life cycles of selected species. Studies of the structure of protistan parasites terization of the parasite species, to elucidation of found in bivalve moll uscs have been fruitful to the many parasite life cycles, and to knowledge of morphologist interested in comparative morphol- parasite metabolism. The latter, especially, is ogy, evolu tion, and taxonomy. -

Shellfish Reefs at Risk
SHELLFISH REEFS AT RISK A Global Analysis of Problems and Solutions Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang CONTENTS Acknowledgments ........................................................................................................................ 1 Executive Summary .................................................................................................................... 2 Introduction .................................................................................................................................. 6 Methods .................................................................................................................................... 10 Results ........................................................................................................................................ 14 Condition of Oyster Reefs Globally Across Bays and Ecoregions ............ 14 Regional Summaries of the Condition of Shellfish Reefs ............................ 15 Overview of Threats and Causes of Decline ................................................................ 28 Recommendations for Conservation, Restoration and Management ................ 30 Conclusions ............................................................................................................................ 36 References ............................................................................................................................. -

Olympia Oyster (Ostrea Lurida)
COSEWIC Assessment and Status Report on the Olympia Oyster Ostrea lurida in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olympia Oyster Ostrea lurida in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 56 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 30 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Gillespie, G.E. 2000. COSEWIC status report on the Olympia Oyster Ostrea conchaphila in Canada in COSEWIC assessment and update status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-30 pp. Production note: COSEWIC acknowledges Graham E. Gillespie for writing the provisional status report on the Olympia Oyster, Ostrea lurida, prepared under contract with Environment Canada and Fisheries and Oceans Canada. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status reports were overseen by Robert Forsyth and Dr. Gerald Mackie, COSEWIC Molluscs Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l’huître plate du Pacifique (Ostrea lurida) au Canada. -

Is Pallial Mucus Involved in Ostrea Edulis Defenses Against the Parasite Bonamia
1 Is pallial mucus involved in Ostrea edulis defenses against the parasite Bonamia 2 ostreae? 3 Sergio Fernández-Boo1,4*, Ophélie Gervais1, Maria Prado-Alvarez3 Bruno Chollet1, 4 Stéphane Claverol2, Cyrielle Lecadet1, Christine Dubreuil1, Isabelle Arzul1 5 1 Institut Français de Recherche pour l´Exploitation de la Mer (IFREMER), Laboratoire de 6 Génétique et Pathologie (LGP), Avenue Mus de Loup, 17390 La Tremblade, France. 7 2 Université de Bordeaux, Centre Génomique Fonctionnelle de Bordeaux, Plateforme 8 Protéome, F-33000 Bordeaux, France. 9 3 Aquatic Molecular Pathobiology Group. Marine Research Institute (IIM-CSIC). Vigo. 10 Spain. 11 4 Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR), University of 12 Porto, Avenida General Norton de Matos, S/N, 4450-208 Matosinhos, Portugal. 13 14 *Corresponding author: [email protected] 15 Abstract 16 Bonamia ostreae is an intrahemocytic parasite that has been responsible for severe 17 mortalities in the flat oyster Ostrea edulis since the 1970´s. The Pacific oyster 18 Crassostrea gigas is considered to be resistant to the disease and appears to have 19 mechanisms to avoid infection. Most studies carried out on the invertebrate immune 20 system focus on the role of hemolymph, although mucus, which covers the body surface 21 of molluscs, could also act as a barrier against pathogens. In this study, the in vitro effect 22 of mucus from the oyster species Ostrea edulis and C. gigas on B. ostreae was 23 investigated using flow cytometry. Results showed an increase in esterase activities and 24 mortality rate of parasites exposed to mucus from both oyster species. -

Chapter I Taxonomy
THE AMERICAN OYSTER CRASSOSTREA VIRGINICA GMELIN By PAUL S. GALTSOFF, Fishery Biologist BUREAU OF COMMERCIAL FISHERIES CHAPTER I TAXONOMY Page This broad characterization included a number Taxonomic characters _ 4 SheIL _ 4 of genera such as scallops, pen shells (Pinnidae), Anatomy _ 4 Sex and spawnlng _ limas (Limidae) and other mollusks which ob 4 Habitat _ 5 viously are not oysters. In the 10th edition of Larvll! shell (Prodlssoconch) _ 6 "Systema Naturae," Linnaeus (1758) wrote: The genera of living oysters _ 6 Genus 08trea _ 6 "Ostreae non orones, imprimis Pectines, ad Genus Cra8808trea _ 7 Genus Pycnodonte _ cardinem interne fulcis transversis numerosis 7 Bibliography _ 14 parallelis in utraque testa oppositis gaudentiquae probe distinguendae ab Areis polypleptoginglymis, The family Ostreidae consists of a large number cujus dentes numerosi alternatim intrant alterius of edibleand nonedible oysters. Their distribution sinus." Le., not all are oysters, in particular the is confined to a broad belt of coastal waters within scallops, which have many parallel ribs running the latitudes 64° N. and 44° S. With few excep crosswise inward toward the hinge on each shell tions oysters thrive in shallow water, their vertical on opposite sides; these should properly be dis distribution extending from a level approximately tinguished from Area polyleptoginglymis whose halfway between high and low tide levels to a many teeth alternately enter between the teeth depth of about 100 feet. Commercially exploited of the other side. oyster beds are rarely found below a depth of 40 In the same publication the European flat feet. oyster, Ostrea edulis, is described as follows: The· name "Ostrea" was given by Linnaeus "Vulgo Ostrea dictae edulis. -

Occurrence and Significance of Bonamiasis in European Flat Oysters Ostrea Edulis in North America
DISEASES OF AQUATIC ORGANISMS Vol. 2: 49-54. 1986 Published December 31 Dis. aquat. Org. l Occurrence and significance of bonamiasis in European flat oysters Ostrea edulis in North America ' Center for Marine Disease Control. BattelleIMarine Research Laboratory, 439 West Sequim Bay Road, Sequim. Washington 98382, USA National Marine Fisheries Service. Northeast Fisheries Center, Oxford, Maryland 21654, USA ABSTRACT: The protozoan blood-cell parasite Bonamia ostreae of the European flat oyster Ostrea edulis has caused extens~ven~ortahties in France, the United Kingdom, The Netherlands, Spain, and Denmark. The discovery of the disease in 4 oyster populations in North America is the flrst documenta- tion of the disease outside Europe. Ultrastructure of the parasite, inflammatory lesions in the affected oysters, and transmissibility patterns of the disease demonstrate the identity of the disease in Europe and North America. Current studies and reconstruction of historical records trace the origin of the disease to California (USA) and possibly an Atlantic North American site, followed by its spread within North America and to Europe, where it was first described in detail and recognized as a significant cause of oyster mortalities. INTRODUCTION prevalance of this significant disease in 9 separate stocks of oysters, and reconstruct historical information A disease leading to extensive mortalities of the which demonstrates the probable route by which this European flat oyster Ostrea eduhs is caused by the serious disease spread within North America and sub- protozoan parasite Bonamia ostreae of the oyster's sequently to Europe. blood cells. First described from oysters in France in 1979 (Comps et al. 1980), significant mortalities of the flat oyster have been attributed to the disease in a METHODS AND MATERIALS variety of European countries including France, Spain, England, Denmark, and The Netherlands (Balouet et A total of 560 flat oysters Ostrea edulis from western al. -

Other Diseases, Other Molluscs
OTHER DISEASES, OTHER MOLLUSCS Hemic Neoplasia of Bivalve Molluscs The disease known as hemic, hematopoietic, or hemocytic neoplasia HCN! is also referred to as hemic proliferative disease, leukocytic neoplasia, sarcomatous neoplasia, sarcomataidproliferative disorder, disseminated sarcoma, and atypical hemocyte condition. As a neoplasia, it is considered ta be a form of cancer of shellfish similar to leukemia in higher animals and man in the way it affects the host. It should be emphasized, however, that this is a cancer of shellfish, not of humans, and that consuming shellfish with this condition poses na known health threat ta humans. Soine research has suggested that the disease is caused by a virus, but this is not yet confirmedor generally accepted. However, it has been shawn in some cases to be highly contagiousfrom one individual shellfish to another. The disease occurs throughout the warld in a variety af bivalve molluscs and appears to causesignificant mortality in certain farmed populations of shellfish. Geographic Range and Species Infected The diseaseaffects many speciesthroughout the world. Like many other shellfish diseases,it is probably more widely distributed than is now known. The following species and locations have been identified: Adula cali fornica, Pacific coast of North America; Artica i slandica mahagany quahog!, Rhode Island Saund, Atlantic caast of Narth America; Ceras- todermaedule common cackle!, Cork Harbour, Ireland; Saccostrea commerciali,s Australian rockoyster!, Australia; Crassostreagigas Japaneseor Pacific oyster!, Matsushima Bay, Japan;Crassostrea rhizophorae, Brazil; CrassostreaUirgi,mica Eastern or American oyster!, Atlantic coast of North America, discontinuously fram the Chesapeake Bay ta Long Island Soundand sites on the Gulf coast; Macoma calcarea, Baffin Island, Canada; Macoma nasuta andM. -

Perspective on Bivalve Mollusc Disease from the Marine Environment
Perspective on Bivalve Mollusc Disease from the Marine Environment Ryan B. Carnegie, PhD Virginia Institute of Marine Science Major Disease Emergence In Bivalve Molluscs Gill iridovirus Bonamia exitiosa Crassostrea angulata Ostrea chilensis France New Zealand MSX disease Crassostrea virginica USA Mid-Atlantic QX disease Malpeque disease Saccostrea glomerata Crassostrea virginica SE Australia Dermo disease Prince Edward Island QPX disease Crassostrea virginica Mercenaria mercenaria USA Mid-Atlantic E. Canada, USA 1910 1920 1930 1940 1950 1960 1970 1980 1990 2000 Herpesviral infection Crassostrea gigas Winter mortality Denman Island disease Marteilia refringens Bonamia ostreae France Saccostrea glomerata Crassostrea gigas Ostrea edulis Ostrea edulis SE Australia British Columbia France France Oyster, Virginia Aubrey Bodine photo, 1960 Strengths of Marine Shellfish Pathology Familiar list of established pathogens and disease agents Effective diagnostic tools for their detection National and international networks of laboratories sharing information on their status, and new disease emergence Deepening pool of biological/ecological knowledge informs management Marteilia FISH for Bonamia Survey site We Have a Good Handle on Who the Pathogens Are Perkinsus marinus Perkinsus, Haplosporidium, Bonamia, Marteilia, Mikrocytos OsHV-1 herpesviruses (presently Haplosporidium nelsoni microvariants thereof) QPX Vibrio, Roseovarius bacteria Or do we? Bonamia exitiosa Vibrio Coming to Terms with Pathogen Diversity New pathogens of concern emerge -

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Category Disease Name Acronym Health Species and group of species Vector Species of Listed Listed National Status Disease Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (Carassius Epizootic Disease-Free Rainbow trout (Oncorhynchus mykiss), redfin carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophthalmichthys haematopoietic EHN A+D+E Status perch (Perca fluviatilis) molitrix), chub (Leuciscus spp.), roach (Rutilus rutilus), rudd (Scardinius erythrophthalmus), necrosis tench (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), sterlet sturgeon (Acipenser ruthenus), starry sturgeon (Acipenser stellatus), sturgeon (Acipenser sturio), Siberian sturgeon (Acipenser baerii), bighead carp (Aristichthys nobilis), goldfish (Carassius Herring (Clupea spp.), whitefish (Coregonus auratus), crucian carp (Carassius carassius), common carp and koi carp (Cyprinus carpio), ssp.), pike (Esox lucius), haddock silver carp (Hypophthalmichthys molitrix), chub (Leuciscus spp.), roach (Rutilus rutilus), rudd Melanogrammus aeglefinus ( ), Pacific cod (Scardinius erythrophthalmus), tench (Tinca tinca), North African catfish (Clarias (Gadus macrocephalus), Atlantic cod (Gadus gariepinus), pike (Esox lucius), catfish (Ictalurus spp.), black bullhead (Ameiurus melas), morhua), Pacific salmon (Oncorhynchus spp.) channel catfish (Ictalurus punctatus), pangas catfish (Pangasius pangasius), pike perch rainbow trout (Oncorhynchus -

Functional Morphology and Development of Veliger Larvae of the European Oyster, Ostrea Edulis Linne
Functional Morphology and Development of Veliger Larvae of the European Oyster, Ostrea edulis Linne THOMAS R. WALLER SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 328 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropo/ogy Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world cf science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review.