Functional Morphology and Development of Veliger Larvae of the European Oyster, Ostrea Edulis Linne
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Long-Duration Anesthetization of Squid (Doryteuthis Pealeii) T
Marine and Freshwater Behaviour and Physiology Vol. 43, No. 4, July 2010, 297–303 Long-duration anesthetization of squid (Doryteuthis pealeii) T. Aran Mooneya,b*, Wu-Jung Leeb and Roger T. Hanlona aMarine Resources Center, Marine Biological Laboratory, Woods Hole, MA 02543, USA; bWoods Hole Oceanographic Institution, Woods Hole, MA 02543, USA (Received 4 May 2010; final version received 15 June 2010) Cephalopods, and particularly squid, play a central role in marine ecosystems and are a prime model animal in neuroscience. Yet, the capability to investigate these animals in vivo has been hampered by the inability to sedate them beyond several minutes. Here, we describe methods to anesthetize Doryteuthis pealeii, the longfin squid, noninvasively for up to 5 h using a 0.15 mol magnesium chloride (MgCl2) seawater solution. Sedation was mild, rapid (54 min), and the duration could be easily controlled by repeating anesthetic inductions. The sedation had no apparent effect on physiological evoked potentials recorded from nerve bundles within the statocyst system, suggesting the suitability of this solution as a sedating agent. This simple, long-duration anesthetic technique opens the possibility for longer in vivo investigations on this and related cephalopods, thus expanding potential neuroethological and ecophysiology research for a key marine invertebrate group. Keywords: anesthesia; sedation; squid; Loligo; neurophysiology; giant axon Introduction Although cephalopods are key oceanic organisms used extensively as experimental animals in a variety of research fields (Gilbert et al. 1990), there is a relative paucity of information on maintaining them under anesthesia for prolonged durations. Lack of established protocols for sedation beyond several minutes constrains Downloaded By: [Hanlon, Roger T.] At: 12:46 19 August 2010 experimental conditions for many neurobiological and physiological preparations. -

Branding for Sabah Pearl 2018
BRANDING FOR SABAH PEARL Tan Yin Yin Bachelor of Applied Arts with Honours (Design Technology) 2018 BRANDII\'G FOR SABAH PEARL 'IlL'1 YII\' YIN Th,s project is submitted in partial fulfillment of the requirements for the degree of Bachelor of Arts with Honors (IlE'sign Technology) Faculty of Apphed and Creative Arts UWVERSITI NiALl, YSIA SARAWAK 2013 PENJENAMAAN UNTUK MUTIARA SABAH TAN \1N \1N Pr'Diekilll merupakan salah satu keperluan untuk ljazah Sarjana Seni Gunaan dengan (Teknologi Sem Reka) Fakulti Seni. Gunaan dan Kreatif UNlVERSITI Mll.Lll. YSIA SARA WAK 2018 ii PIE'.u~ (leI> ( 'd FUll.! \'t","\r ?r -:~ <:' 1 R~pc.n :J :·::: ~r s PhD O(C'L\.R:HIO:--: Of ORIGI);_\L \,"ORb. dar ot ._ ;':II I S .Studt-nt's Decl"l'.1(1oD r . ICI"YtYl)!h (J 1f O. ~7.)~m fa(,tlt'r° fAr p.ii~4~f). (((r.e~ ~y'e, Arts IPLZ-l..t: =: e\"Dl8::..r ::: ~~ l·D ~:\T~~,.'\.:J:::. :-"L';'IRJC:--:O :1...'\-n f..I,.C"""L n"! htrebr dtcl:m' ~hat the wcrt tr~m i l'd .. [a.(!d.t' .J..r ~ r. ,;. o::Mh:. ... P..e.~ .d ...... _......... _..... ................... !"i Ill)" " n~l!Ji\ l we.rk I han: c o, Or:'le- j n- ot::. ilDr othH S'tud .. n;:~ wort vr trow any other sourcE' __ t :': c~ p: \\h~lt dut refH t'o{'e or lC' t.no":lt'd~tI1l ..nt!i ~l dt t::pJ.i.C1tl~ · t.n [he te:;:l on ha~ anr part been ';\,III('n for Ill t by " o. -

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -

Colored Gemstones Cultured Pearls
Cultured Pearls Colored Gemstones Diamond Council of America ©2016 Cultured Pearls In This Lesson: •A World Apart • Pearl Traditions • Natural Pearls • Cultured Pearls •Value Factors •Product Highlights • Culturing Sales A WORLD APART In Lesson 1 you learned that any kind of gem except diamond is considered a colored gem. Although pearls are included in that broad classification, they really belong to a world apart. Most customers recognize this instinctively, sensing a special appeal about pearls. There are several themes you can use in a sales presenta- tion to evoke or enhance pearl’s separate place in the gem kingdom: • Pearls are born in water. This intuitive contrast with other gems, which are dug from the ground, gives pearls an aura of gentleness, freshness, and fluid grace. • Pearls originate from life. While most gems are minerals produced by inanimate geology, pearls are organic. They come from living beings. Much of pearls’ mystique arises from this connection. • Pearls possess a beauty that’s all their own. Most gems depend on cutting or carving to reveal their charms, but pearls emerge gleaming from their shells. Cultured pearls are born in water and originate from living organisms. They Though certain factors of pearl value are comparable are natural in their beauty and classic to those of other gems, key considerations are unique. as a gem. Colored Gemstones 5 1 Cultured Pearls Cultured pearls are modern forms of a classic gem. They ® combine Nature’s creative power with human art and JA SPC SKILLS If you’re participating in the JA® science. You could even say that cultured pearls show how Sales Professional Certification people can work with the environment to make age-old Program™, this lesson presents infor- mation related to the following Skill beauty available now, and for future generations as well. -

Impacts of Ocean Acidification on Marine Shelled Molluscs
Mar Biol DOI 10.1007/s00227-013-2219-3 ORIGINAL PAPER Impacts of ocean acidification on marine shelled molluscs Fre´de´ric Gazeau • Laura M. Parker • Steeve Comeau • Jean-Pierre Gattuso • Wayne A. O’Connor • Sophie Martin • Hans-Otto Po¨rtner • Pauline M. Ross Received: 18 January 2013 / Accepted: 15 March 2013 Ó Springer-Verlag Berlin Heidelberg 2013 Abstract Over the next century, elevated quantities of ecosystem services including habitat structure for benthic atmospheric CO2 are expected to penetrate into the oceans, organisms, water purification and a food source for other causing a reduction in pH (-0.3/-0.4 pH unit in the organisms. The effects of ocean acidification on the growth surface ocean) and in the concentration of carbonate ions and shell production by juvenile and adult shelled molluscs (so-called ocean acidification). Of growing concern are the are variable among species and even within the same impacts that this will have on marine and estuarine species, precluding the drawing of a general picture. This organisms and ecosystems. Marine shelled molluscs, which is, however, not the case for pteropods, with all species colonized a large latitudinal gradient and can be found tested so far, being negatively impacted by ocean acidifi- from intertidal to deep-sea habitats, are economically cation. The blood of shelled molluscs may exhibit lower and ecologically important species providing essential pH with consequences for several physiological processes (e.g. respiration, excretion, etc.) and, in some cases, increased mortality in the long term. While fertilization Communicated by S. Dupont. may remain unaffected by elevated pCO2, embryonic and Fre´de´ric Gazeau and Laura M. -

Microchemistry of Juvenile Mercenaria Mercenaria Shell: Implications for Modeling Larval Dispersal
Vol. 465: 155–168, 2012 MARINE ECOLOGY PROGRESS SERIES Published September 28 doi: 10.3354/meps09895 Mar Ecol Prog Ser Microchemistry of juvenile Mercenaria mercenaria shell: implications for modeling larval dispersal A. M. Cathey1,*, N. R. Miller2, D. G. Kimmel3 1Department of Biology, East Carolina University, Greenville, North Carolina 27858, USA 2Department of Geological Sciences, The University of Texas at Austin, Austin, Texas 78712, USA 3Department of Biology, Institute for Coastal Science and Policy, East Carolina University, Greenville, North Carolina 27858, USA ABSTRACT: The elemental signature of trace and minor elements within biominerals has been used to investigate the larval dispersal of bivalves. We investigated the potential of this technique by examining elemental signals in juvenile shells of the hard clam Mercenaria mercenaria within an estuarine−lagoonal system near Cape Lookout, North Carolina, USA. We assessed the spatial distinction (~12 to 40 km) and temporal stability (fall versus spring) of elemental concentrations using inductively coupled plasma mass spectrometry (ICP-MS). Twelve minor and trace elements were present at detectable levels in all shell samples: calcium (Ca), manganese (Mn), aluminum (Al), titanium (Ti), cobalt (Co), copper (Cu), barium (Ba), magnesium (Mg), zinc (Zn), lead (Pb), nickel (Ni), and strontium (Sr). Discriminant function analyses (DFA) using metal to Ca ratios as independent variables correctly assigned hard clams to their site of collection with 100% success from both fall and spring sampling events. Mn:Ca ratio proved to be the most effective discrimi- nator explaining 91.2 and 71.9% of our among-group variance, respectively. Elemental concentra- tions within juvenile shell differed temporally. -
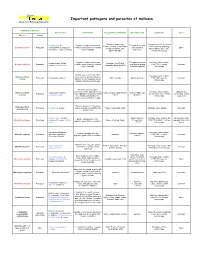
Pathogens Table 2006
Important pathogens and parasites of molluscs Genetic and Pathology Laboratory Pathogen or parasite Host species Host impact Geographical distribution Infection period Diagnostic Cycle Species Group Denmark, Netherland, Incubation period : 3-4 month Ostrea edulis, O. Parasite of oyster haemocytes Throughout the year France, Ireland, Great Britain in infected area. Histology, Bonamia ostreae Protozoan Conchaphila, O. Angasi, O. (=>all the tissues can be invaded). (with a peak in Direct (except Scotland), Italy, tissue imprint, PCR, ISH, Puelchana, Tiostrea chilensis Oyster mortality September) Spain and USA electron microscopy Parasite of oyster haemocytes Throughout the year Histology, tissue imprint, Ostrea angasi, Ostrea Australia, New Zéland, Bonamia exitiosa Protozoan (=>all the tissues can be invaded). (with a peak during PCR, ISH, electron Unknown chilensis, Ostrea edulis Tasmania, Spain (in 2007) Oyster mortality Australian autumn) microscopy Parasites present in connective Histology, tissue imprint, Haplosporidium tissue (mantle, gonads, digestive Protozoan Crassostrea virginica USA, Canada Spring-summer PCR, ISH, electron Unknown costale gland) but not in digestive tubule microscopy epithelia. Mortality in May-June Parasites present in gills, connective tissue. Sporulation only Histology, tissue imprint, Unknown but Haplosporidium Crassostrea virginica, USA, Canada, Japan, Korea, Summer (May until Protozoan in the epithelium of digestive gland smear, PCR, HIS, electron intermediate host is nelsoni Crassostrea gigas France October) in C. virginica, Mortalities in C. microscopy suspected virginica. No mortality in C. gigas Parasite present in connective Haplosporidium Protozoan O. edulis, O. angasi tissue. Sometimes, mortality can France, Netherland, Spain Histology, tissue imprint Unknown armoricanum be observed Ostrea edulis , Tiostrea Spring-summer Histology, tissue imprint, ISH, Intermediate host: Extracellular parasite of the Marteilia refringens Protozoan chilensis, O. -

Structure of Protistan Parasites Found in Bivalve Molluscs
W&M ScholarWorks VIMS Books and Book Chapters Virginia Institute of Marine Science 1988 Structure of Protistan Parasites Found in Bivalve Molluscs Frank O. Perkins Follow this and additional works at: https://scholarworks.wm.edu/vimsbooks Part of the Marine Biology Commons, and the Parasitology Commons American Fisheries Society Special Publication 18:93- 111 , 1988 CC> Copyrighl by !he American Fisheries Sociely 1988 PARASITE MORPHOLOGY, STRATEGY, AND EVOLUTION Structure of Protistan Parasites Found in Bivalve Molluscs 1 FRANK 0. PERKINS Virginia In stitute of Marine Science. School of Marine Science, College of William and Mary Gloucester Point, Virginia 23062, USA Abstral'I.-The literature on the structure of protists parasitizing bivalve molluscs is reviewed, and previously unpubli shed observations of species of class Perkinsea, phylum Haplosporidia, and class Paramyxea are presented. Descriptions are given of the flagellar apparatus of Perkin.His marinus zoospores, the ultrastructure of Perkinsus sp. from the Baltic macoma Maconw balthica, and the development of haplosporosome-like bodies in Haplosporidium nelsoni. The possible origin of stem cells of Marreilia sydneyi from the inner two sporoplasms is discussed. New research efforts are suggested which could help elucidate the phylogenetic interrelationships and taxonomic positions of the various taxa and help in efforts to better understand life cycles of selected species. Studies of the structure of protistan parasites terization of the parasite species, to elucidation of found in bivalve moll uscs have been fruitful to the many parasite life cycles, and to knowledge of morphologist interested in comparative morphol- parasite metabolism. The latter, especially, is ogy, evolu tion, and taxonomy. -

Shellfish Reefs at Risk
SHELLFISH REEFS AT RISK A Global Analysis of Problems and Solutions Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang CONTENTS Acknowledgments ........................................................................................................................ 1 Executive Summary .................................................................................................................... 2 Introduction .................................................................................................................................. 6 Methods .................................................................................................................................... 10 Results ........................................................................................................................................ 14 Condition of Oyster Reefs Globally Across Bays and Ecoregions ............ 14 Regional Summaries of the Condition of Shellfish Reefs ............................ 15 Overview of Threats and Causes of Decline ................................................................ 28 Recommendations for Conservation, Restoration and Management ................ 30 Conclusions ............................................................................................................................ 36 References ............................................................................................................................. -

Histopathology of Oedema in Pearl Oysters Pinctada Maxima
Vol. 91: 67–73, 2010 DISEASES OF AQUATIC ORGANISMS Published July 26 doi: 10.3354/dao02229 Dis Aquat Org Histopathology of oedema in pearl oysters Pinctada maxima J. B. Jones*, M. Crockford, J. Creeper, F. Stephens Department of Fisheries, PO Box 20, North Beach, Western Australia 6920, Australia ABSTRACT: In October 2006, severe mortalities (80 to 100%) were reported in pearl oyster Pinctada maxima production farms from Exmouth Gulf, Western Australia. Only P. maxima were affected; other bivalves including black pearl oysters P. margaratifera remained healthy. Initial investigations indicated that the mortality was due to an infectious process, although no disease agent has yet been identified. Gross appearance of affected oysters showed mild oedema, retraction of the mantle, weak- ness and death. Histology revealed no inflammatory response, but we did observe a subtle lesion involving tissue oedema and oedematous separation of epithelial tissues from underlying stroma. Oedema or a watery appearance is commonly reported in published descriptions of diseased mol- luscs, yet in many cases the terminology has been poorly characterised. The potential causes of oedema are reviewed; however, the question remains as to what might be the cause of oedema in molluscs that are normally iso-osmotic with seawater and have no power of anisosmotic extracellular osmotic regulation. KEY WORDS: Oedema · Pinctada maxima · Osmosis · Lesion · Mortality Resale or republication not permitted without written consent of the publisher INTRODUCTION the threat of disease and a complex series of transport protocols and separation of pearl farm lease areas The annual value of production of the pearling has been required (Jones 2008). -

Octopus Consciousness: the Role of Perceptual Richness
Review Octopus Consciousness: The Role of Perceptual Richness Jennifer Mather Department of Psychology, University of Lethbridge, Lethbridge, AB T1K 3M4, Canada; [email protected] Abstract: It is always difficult to even advance possible dimensions of consciousness, but Birch et al., 2020 have suggested four possible dimensions and this review discusses the first, perceptual richness, with relation to octopuses. They advance acuity, bandwidth, and categorization power as possible components. It is first necessary to realize that sensory richness does not automatically lead to perceptual richness and this capacity may not be accessed by consciousness. Octopuses do not discriminate light wavelength frequency (color) but rather its plane of polarization, a dimension that we do not understand. Their eyes are laterally placed on the head, leading to monocular vision and head movements that give a sequential rather than simultaneous view of items, possibly consciously planned. Details of control of the rich sensorimotor system of the arms, with 3/5 of the neurons of the nervous system, may normally not be accessed to the brain and thus to consciousness. The chromatophore-based skin appearance system is likely open loop, and not available to the octopus’ vision. Conversely, in a laboratory situation that is not ecologically valid for the octopus, learning about shapes and extents of visual figures was extensive and flexible, likely consciously planned. Similarly, octopuses’ local place in and navigation around space can be guided by light polarization plane and visual landmark location and is learned and monitored. The complex array of chemical cues delivered by water and on surfaces does not fit neatly into the components above and has barely been tested but might easily be described as perceptually rich. -

Early Ontogeny of Jurassic Bakevelliids and Their Bearing on Bivalve Evolution
Early ontogeny of Jurassic bakevelliids and their bearing on bivalve evolution NIKOLAUS MALCHUS Malchus, N. 2004. Early ontogeny of Jurassic bakevelliids and their bearing on bivalve evolution. Acta Palaeontologica Polonica 49 (1): 85–110. Larval and earliest postlarval shells of Jurassic Bakevelliidae are described for the first time and some complementary data are given concerning larval shells of oysters and pinnids. Two new larval shell characters, a posterodorsal outlet and shell septum are described. The outlet is homologous to the posterodorsal notch of oysters and posterodorsal ridge of arcoids. It probably reflects the presence of the soft anatomical character post−anal tuft, which, among Pteriomorphia, was only known from oysters. A shell septum was so far only known from Cassianellidae, Lithiotidae, and the bakevelliid Kobayashites. A review of early ontogenetic shell characters strongly suggests a basal dichotomy within the Pterio− morphia separating taxa with opisthogyrate larval shells, such as most (or all?) Praecardioida, Pinnoida, Pterioida (Bakevelliidae, Cassianellidae, all living Pterioidea), and Ostreoida from all other groups. The Pinnidae appear to be closely related to the Pterioida, and the Bakevelliidae belong to the stem line of the Cassianellidae, Lithiotidae, Pterioidea, and Ostreoidea. The latter two superfamilies comprise a well constrained clade. These interpretations are con− sistent with recent phylogenetic hypotheses based on palaeontological and genetic (18S and 28S mtDNA) data. A more detailed phylogeny is hampered by the fact that many larval shell characters are rather ancient plesiomorphies. Key words: Bivalvia, Pteriomorphia, Bakevelliidae, larval shell, ontogeny, phylogeny. Nikolaus Malchus [[email protected]], Departamento de Geologia/Unitat Paleontologia, Universitat Autòno− ma Barcelona, 08193 Bellaterra (Cerdanyola del Vallès), Spain.