Diseases Affecting Finfish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WHELKS Scientific Names: Busycon Canaliculatum Busycon Carica
Colloquial Nicknames: Channeled Whelk Knobbed Whelk WHELKS Scientific names: Busycon canaliculatum Busycon carica Field Markings: The shell of open with their strong muscular foot. As both species is yellow-red or soon as the valves open, even the tiniest orange inside and pale gray amount, the whelk wedges in the sharp edge outside. of its shell, inserts the proboscis and Size: Channeled whelk grows up devours the soft body of the clam. to 8 inches long; knobbed whelk Mating occurs by way of internal grows up to 9 inches long and 4.5 inches wide fertilization; sexes are separate. The egg casing of the whelk is a Habitat: Sandy or muddy bottoms long strand of yellowish, parchment-like disks, resembling a Seasonal Appearance: Year-round necklace - its unique shape is sculpted by the whelk’s foot. Egg cases can be two to three feet long and have 70 to 100 capsules, DISTINGUISHING FEATURES AND each of which can hold 20 to 100 eggs. Newly hatched channeled BEHAVIORS whelks escape from small holes at the top of each egg case with Whelks are large snails with massive shells. The two most their shells already on. Egg cases are sometimes found along common species in Narragansett Bay are the knobbed whelk the Bay shoreline, washed up with the high tide debris. and the channeled whelk. The knobbed whelk is the largest marine snail in the Bay. It Relationship to People is pear-shaped with a flared outer lip and knobs on the shoulder Both channeled and knobbed whelks scavenge and hunt for of its shell. -

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -

National Review of Ostrea Angasi Aquaculture: Historical Culture, Current Methods and Future Priorities
National review of Ostrea angasi aquaculture: historical culture, current methods and future priorities Christine Crawford Institute of Marine and Antarctic Studies ! [email protected] " secure.utas.edu.au/profles/staff/imas/Christine-Crawford Executive summary Currently in Australia Ostrea angasi oysters (angasi) are being cultured on a small scale, with several farmers growing relatively small numbers of angasis on their predominately Sydney rock or Pacifc oyster farms. Very few farmers are culturing commercially viable quantities of angasi oysters. There are several reasons for this. Although angasi oysters occur in the intertidal zone, they are naturally most abundant in the subtidal and are less tolerant of fuctuating environmental conditions, especially temperature and salinity, than other oyster species. They also have a much shorter shelf life and start to gape after one to two days. Additionally, angasi oysters are susceptible to Bonamiosis, a parasitic disease which has caused major mortalities in several areas. Stress caused by extremes or a combination of factors such as high stocking densities, rough handling, poor food, high temperatures and low salinities have all been observed to increase the prevalence of Bonamiosis. Growth rates of angasi oysters have also been variable, ranging from two to four years to reach market size. From discussions with oyster famers, managers and researchers and from a review of the literature I suggest that the survival and growth of cultured angasi oysters could be signifcantly improved by altering some farm management practices. Firstly, growout techniques need to be specifcally developed for angasi oysters which maintain a low stress environment (not modifcations from other oysters). -
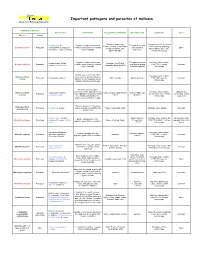
Pathogens Table 2006
Important pathogens and parasites of molluscs Genetic and Pathology Laboratory Pathogen or parasite Host species Host impact Geographical distribution Infection period Diagnostic Cycle Species Group Denmark, Netherland, Incubation period : 3-4 month Ostrea edulis, O. Parasite of oyster haemocytes Throughout the year France, Ireland, Great Britain in infected area. Histology, Bonamia ostreae Protozoan Conchaphila, O. Angasi, O. (=>all the tissues can be invaded). (with a peak in Direct (except Scotland), Italy, tissue imprint, PCR, ISH, Puelchana, Tiostrea chilensis Oyster mortality September) Spain and USA electron microscopy Parasite of oyster haemocytes Throughout the year Histology, tissue imprint, Ostrea angasi, Ostrea Australia, New Zéland, Bonamia exitiosa Protozoan (=>all the tissues can be invaded). (with a peak during PCR, ISH, electron Unknown chilensis, Ostrea edulis Tasmania, Spain (in 2007) Oyster mortality Australian autumn) microscopy Parasites present in connective Histology, tissue imprint, Haplosporidium tissue (mantle, gonads, digestive Protozoan Crassostrea virginica USA, Canada Spring-summer PCR, ISH, electron Unknown costale gland) but not in digestive tubule microscopy epithelia. Mortality in May-June Parasites present in gills, connective tissue. Sporulation only Histology, tissue imprint, Unknown but Haplosporidium Crassostrea virginica, USA, Canada, Japan, Korea, Summer (May until Protozoan in the epithelium of digestive gland smear, PCR, HIS, electron intermediate host is nelsoni Crassostrea gigas France October) in C. virginica, Mortalities in C. microscopy suspected virginica. No mortality in C. gigas Parasite present in connective Haplosporidium Protozoan O. edulis, O. angasi tissue. Sometimes, mortality can France, Netherland, Spain Histology, tissue imprint Unknown armoricanum be observed Ostrea edulis , Tiostrea Spring-summer Histology, tissue imprint, ISH, Intermediate host: Extracellular parasite of the Marteilia refringens Protozoan chilensis, O. -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

Structure of Protistan Parasites Found in Bivalve Molluscs
W&M ScholarWorks VIMS Books and Book Chapters Virginia Institute of Marine Science 1988 Structure of Protistan Parasites Found in Bivalve Molluscs Frank O. Perkins Follow this and additional works at: https://scholarworks.wm.edu/vimsbooks Part of the Marine Biology Commons, and the Parasitology Commons American Fisheries Society Special Publication 18:93- 111 , 1988 CC> Copyrighl by !he American Fisheries Sociely 1988 PARASITE MORPHOLOGY, STRATEGY, AND EVOLUTION Structure of Protistan Parasites Found in Bivalve Molluscs 1 FRANK 0. PERKINS Virginia In stitute of Marine Science. School of Marine Science, College of William and Mary Gloucester Point, Virginia 23062, USA Abstral'I.-The literature on the structure of protists parasitizing bivalve molluscs is reviewed, and previously unpubli shed observations of species of class Perkinsea, phylum Haplosporidia, and class Paramyxea are presented. Descriptions are given of the flagellar apparatus of Perkin.His marinus zoospores, the ultrastructure of Perkinsus sp. from the Baltic macoma Maconw balthica, and the development of haplosporosome-like bodies in Haplosporidium nelsoni. The possible origin of stem cells of Marreilia sydneyi from the inner two sporoplasms is discussed. New research efforts are suggested which could help elucidate the phylogenetic interrelationships and taxonomic positions of the various taxa and help in efforts to better understand life cycles of selected species. Studies of the structure of protistan parasites terization of the parasite species, to elucidation of found in bivalve moll uscs have been fruitful to the many parasite life cycles, and to knowledge of morphologist interested in comparative morphol- parasite metabolism. The latter, especially, is ogy, evolu tion, and taxonomy. -

Shellfish Reefs at Risk
SHELLFISH REEFS AT RISK A Global Analysis of Problems and Solutions Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang CONTENTS Acknowledgments ........................................................................................................................ 1 Executive Summary .................................................................................................................... 2 Introduction .................................................................................................................................. 6 Methods .................................................................................................................................... 10 Results ........................................................................................................................................ 14 Condition of Oyster Reefs Globally Across Bays and Ecoregions ............ 14 Regional Summaries of the Condition of Shellfish Reefs ............................ 15 Overview of Threats and Causes of Decline ................................................................ 28 Recommendations for Conservation, Restoration and Management ................ 30 Conclusions ............................................................................................................................ 36 References ............................................................................................................................. -

Optimization of in Vitro Fertilization Using Cryopreserved Sperm of Crassostrea Angulata and Establishment of a Cryopreservation Protocol for Chamelea Gallina
Ana Luísa Lopes Santos Optimization of in vitro fertilization using cryopreserved sperm of Crassostrea angulata and establishment of a cryopreservation protocol for Chamelea gallina UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS E TECNOLOGIA 2018 Ana Luísa Lopes Santos Optimization of in vitro fertilization using cryopreserved sperm of Crassostrea angulata and establishment of a cryopreservation protocol for Chamelea gallina Thesis for Master Degree in Aquaculture and Fisheries Specialization Aquaculture Thesis supervision by: Professora Doutora Elsa Cabrita, CCMAR, Universidade do Algarve Dra Catarina Anjos, CCMAR, Universidade do Algarve UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS E TECNOLOGIA 2018 ii “Declaro ser a autora deste trabalho, que é original e inédito. Autores e trabalhos consultados estão devidamente citados no texto e constam da listagem de referências incluída.” ________________________________________________ Ana Luísa Lopes Santos Copyright © “A Universidade do Algarve tem o direito, perpétuo e sem limites geográficos, de arquivar e publicitar este trabalho através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, de o divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objetivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor.” iii Eles não sabem, nem sonham, que o sonho comanda a vida, que sempre que um homem sonha o mundo pula e avança como bola colorida entre as mãos de uma criança.” António Gedeão iv This work was supported by VENUS Project (0139_VENUS_5_E) “Estudio Integral de los bancos naturales de moluscos bivalvos en el Golfo de Cádiz para su gestión sostenible y la conservación de sus hábitats asociados”. -

Age, Growth, Size at Sexual Maturity and Reproductive Biology of Channeled Whelk, Busycotypus Canaliculatus, in the U.S. Mid-Atlantic
Age, Growth, Size at Sexual Maturity and Reproductive Biology of Channeled Whelk, Busycotypus canaliculatus, in the U.S. Mid-Atlantic October 2015 Robert A. Fisher Virginia Institute of Marine Science Virginia Sea Grant-Affiliated Extension (In cooperation with Bernie’s Conchs) Robert A. Fisher Marine Advisory Services Virginia Institute of Marine Science P.O. Box 1346 Gloucester Point, VA 23062 804/684-7168 [email protected] www.vims.edu/adv VIMS Marine Resource Report No. 2015-15 VSG-15-09 Additional copies of this publication are available from: Virginia Sea Grant Communications Virginia Institute of Marine Science P.O. Box 1346 Gloucester Point, VA 23062 804/684-7167 [email protected] Cover Photo: Robert Fisher, VIMS MAS This work is affiliated with the Virginia Sea Grant Program, by NOAA Office of Sea Grant, U.S. Depart- ment of Commerce, under Grant No. NA10OAR4170085. The views expressed herein do not necessar- ily reflect the views of any of those organizations. Age, Growth, Size at Sexual Maturity and Reproductive Biology of Channeled Whelk, Busycotypus canaliculatus, in the U.S. Mid-Atlantic Final Report for the Virginia Fishery Resource Grant Program Project 2009-12 Abstract The channeled whelk, Busycotypus canaliculatus, was habitats, though mixing is observed inshore along shallow sampled from three in-shore commercially harvested waters of continental shelf. Channeled whelks are the resource areas in the US Mid-Atlantic: off Ocean City, focus of commercial fisheries throughout their range (Davis Maryland (OC); Eastern Shore of Virginia (ES); and and Sisson 1988, DiCosimo 1988, Bruce 2006, Fisher and Virginia Beach, Virginia (VB). -

Olympia Oyster (Ostrea Lurida)
COSEWIC Assessment and Status Report on the Olympia Oyster Ostrea lurida in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olympia Oyster Ostrea lurida in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 56 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 30 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Gillespie, G.E. 2000. COSEWIC status report on the Olympia Oyster Ostrea conchaphila in Canada in COSEWIC assessment and update status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-30 pp. Production note: COSEWIC acknowledges Graham E. Gillespie for writing the provisional status report on the Olympia Oyster, Ostrea lurida, prepared under contract with Environment Canada and Fisheries and Oceans Canada. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status reports were overseen by Robert Forsyth and Dr. Gerald Mackie, COSEWIC Molluscs Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l’huître plate du Pacifique (Ostrea lurida) au Canada. -

Assessment of the Aquacultural Potential of the Portuguese Oyster Crassostrea Angulata
Assessment of the aquacultural potential of the Portuguese oyster Crassostrea angulata Frederico Miguel Mota Batista Dissertação de doutoramento em Ciências do Meio Aquático 2007 Frederico Miguel Mota Batista Assessment of the aquacultural potential of the Portuguese oyster Crassostrea angulata Dissertação de Candidatura ao grau de Doutor em Ciências do Meio Aquático submetida ao Instituto de Ciências Biomédicas de Abel Salazar da Universidade do Porto. Orientador – Doutor Pierre Boudry Categoria – Investigador Afiliação – “Institut Français de Recherche pour l’Exploitation de la Mer” Co-orientador – Professora Doutora Maria Armanda Henriques Categoria –Professora catedrática Afiliação – Instituto de Ciências Biomédicas Abel Salazar da Universidade do Porto. ACKNOWLEDGEMENTS First of all, I would like to thank Dr. Pierre Boudry for accepting to supervise this work, for your commitment and for providing me all conditions that made possible this work. Thank you also for your guidance and at the same time for giving me the freedom to make my own decisions which helped me to become a more independent researcher. Gostaria de agradecer à minha co-orientadora, a Professora Doutora Maria Armanda Henriques por me ter aceite como seu aluno de doutoramento e pela disponibilidade. Gostaria de agradecer ao Dr. Carlos Costa Monteiro por ter permitido a realização de parte deste trabalho na estação experimental de moluscicultura de Tavira do Instituto Nacional de Investigação Agrária e das Pescas (INIAP/IPIMAR). Gostaria também de agradecer-lhe pela disponibilidade e incentivo durante o desenrolar do doutoramento. Je remercie Dr. Philippe Goulletquer et Dr. Tristan Renault pour m’avoir accueillie au sein du Laboratoire de Génétique et Pathologie de la Station de La Tremblade de l’Institut Français de Recherche pour l’Exploitation de la Mer (IFREMER). -

Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi Alvar Carranza, Loren D
Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, M atthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang Results. Condition of Oyster Reefs Globally Across Bays and Ecoregions. Regional Summaries of the Condition of Shellfish Reefs Overview of Threats and Causes of Decline. Recommendations for Conservation, Restoration and Management Conclusions References Appendix 1 Michael W. Beck“, Robert D. Brumbaughb, Laura AiroldL, Alvar Carranzad, Loren D. Coen*, Christine Crawfordi Omar Defeod, Graham J. Edgarf, Boze Hancock®, Matthew Kayh, Hunter Lenihan11, Mark W. Luckenbach', Caitlyn L. Toropova“, Guofan Zhang “ The Nature Conservancy, Institute of Marine Sciences, University of California, Santa Cruz, CA, 95060 b b The Nature Conservancy; PO Box 420237, Summerland Key, FL 33042 * Dipartimento di Biología Evoluziomstica Sperimentale, Université di Bologna, Via S. Alberto 163,1-48100 Ravenna, Italy d d Marine Science Unit, Ecology Department, Faculty of Sciences, Montevideo, Uruguay * Sanibel-Captiva Conservation Foundation, 9 0 0 A Tarpon Bay Road, Sanibel, FL 33957 f Tasmanian Aquaculture and Fisheries Institute, University of Tasmania, Hobart, Tasmania, Australia ® The Nature Conservancy; University of Rhode Island, Narragansett, Rl 028882 h Bren School, University of California, Santa Barbara, CA 93106-5131 ' Virginia Institute of Marine Science, College of William and Mary, Wachapreague, VA 23480 i Institute of Oceanology, Chinese Academy of Sciences, Qingdao, Shandong 266071, China Cover photo: Oyster reets at Virginia Coastal Reserve. © Barry T ru itt/T N C © Barry T ru itt/T N C Many colleagues contributed to this assessment by The authors in particular thank Christine Shepard, Zach providing access to data sets ranging from local to global Ferdaña, Jeff Vincent, Antonella Fatone, Ximing Guo, and scales, helping to find important and often obscure Bill Arnold for help with the data, figures and maps.