CASE REPORTS for the OSPAR LIST of THREATENED And/Or DECLINING SPECIES and HABITATS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fishing and Illegal Trade of the Angelshark DNA Barcoding
Fisheries Research 206 (2018) 193–197 Contents lists available at ScienceDirect Fisheries Research journal homepage: www.elsevier.com/locate/fishres The fishing and illegal trade of the angelshark: DNA barcoding against T misleading identifications ⁎ Ingrid Vasconcellos Bunholia, Bruno Lopes da Silva Ferrettea,b, , Juliana Beltramin De Biasia,b, Carolina de Oliveira Magalhãesa,b, Matheus Marcos Rotundoc, Claudio Oliveirab, Fausto Forestib, Fernando Fernandes Mendonçaa a Laboratório de Genética Pesqueira e Conservação (GenPesC), Instituto do Mar (IMar), Universidade Federal de São Paulo (UNIFESP), Santos, SP, 11070-102, Brazil b Laboratório de Biologia e Genética de Peixes (LBGP), Instituto de Biociências de Botucatu (IBB), Universidade Estadual Paulista (UNESP), Botucatu, SP, 18618-689, Brazil c Acervo Zoológico da Universidade Santa Cecília (AZUSC), Universidade Santa Cecília (Unisanta), Santos, SP, 11045-907, Brazil ARTICLE INFO ABSTRACT Handled by J Viñas Morphological identification in the field can be extremely difficult considering fragmentation of species for trade Keywords: or high similarity between congeneric species. In this context, the shark group belonging to the genus Squatina is Conservation composed of three species distributed in the southern part of the western Atlantic. These three species are Endangered species classified in the IUCN Red List as endangered, and they are currently protected under Brazilian law, which Fishing monitoring prohibits fishing and trade. Molecular genetic tools are now used for practical taxonomic identification, parti- Forensic genetics cularly in cases where morphological observation is prevented, e.g., during fish processing. Consequently, DNA fi Mislabeling identi cation barcoding was used in the present study to track potential crimes against the landing and trade of endangered species along the São Paulo coastline, in particular Squatina guggenheim (n = 75) and S. -

Fisheries Centre
Fisheries Centre The University of British Columbia Working Paper Series Working Paper #2015 - 80 Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulman, Adib Saad, Kyrstn Zylich, Daniel Pauly and Dirk Zeller Year: 2015 Email: [email protected] This working paper is made available by the Fisheries Centre, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada. Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulmana, Adib Saadb, Kyrstn Zylicha, Daniel Paulya, Dirk Zellera a Sea Around Us, Fisheries Centre, University of British Columbia, 2202 Main Mall, Vancouver, BC, V6T 1Z4, Canada b President of Syrian National Committee for Oceanography, Tishreen University, Faculty of Agriculture, P.O. BOX; 1408, Lattakia, Syria [email protected] (corresponding author); [email protected]; [email protected]; [email protected]; [email protected] ABSTRACT Syria’s total marine fisheries catches were estimated for the 1950-2010 time period using a reconstruction approach which accounted for all fisheries removals, including unreported commercial landings, discards, and recreational and subsistence catches. All unreported estimates were added to the official data, as reported by the Syrian Arab Republic to the United Nation’s Food and Agriculture Organization (FAO). Total reconstructed catch for 1950-2010 was around 170,000 t, which is 78% more than the amount reported by Syria to the FAO as their national catch. The unreported components added over 74,000 t of unreported catches, of which 38,600 t were artisanal landings, 16,000 t industrial landings, over 4,000 t recreational catches, 3,000 t subsistence catches and around 12,000 t were discards. -

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -

Species Almanac • Nature Activities At
The deeriNature Almanac What is the i in deeriNature? Is it information, internet? How about identification. When you go out on the Deer Isle preserves, what species are you almost certain to encounter? Which ones might you wish to identify? Then how do you organize your experience so that learning about the nearly overwhelming richness of nature becomes wonderfully satisfying? A century ago every farmer, medicine woman, and indeed any educated man or woman felt that they should have a solid knowledge of the plants around them. The Fairbanks Museum in St. Johnsbury, Vermont has maintained a Flower Table with labeled specimens since 1905. The Deer Isle-Stonington Historical Society has an antique herbarium collection made by Ada Southworth, a Dunham’s point rusticator. Today there are lovely field guides galore but the equivalent of a local list can come to you now by digital download. Here is an almanac, a list of likely plant and animal species (and something about rocks too) for our Deer Isle preserves, arranged according to season and habitat. Enjoy this free e-Book on your desktop, tablet or smartphone. Take this e-book with you on the trails and consult the Point of Interest signs. If you have a smartphone and adequate coverage, at some preserves a QR code will tell you more at the Points of Interest. After each category on the lists you will find suggestions for books to consult or acquire. You will have to read the on line reviews for apps as that field is developing too rapidly for any other approach. -
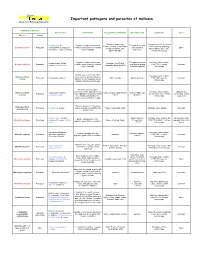
Pathogens Table 2006
Important pathogens and parasites of molluscs Genetic and Pathology Laboratory Pathogen or parasite Host species Host impact Geographical distribution Infection period Diagnostic Cycle Species Group Denmark, Netherland, Incubation period : 3-4 month Ostrea edulis, O. Parasite of oyster haemocytes Throughout the year France, Ireland, Great Britain in infected area. Histology, Bonamia ostreae Protozoan Conchaphila, O. Angasi, O. (=>all the tissues can be invaded). (with a peak in Direct (except Scotland), Italy, tissue imprint, PCR, ISH, Puelchana, Tiostrea chilensis Oyster mortality September) Spain and USA electron microscopy Parasite of oyster haemocytes Throughout the year Histology, tissue imprint, Ostrea angasi, Ostrea Australia, New Zéland, Bonamia exitiosa Protozoan (=>all the tissues can be invaded). (with a peak during PCR, ISH, electron Unknown chilensis, Ostrea edulis Tasmania, Spain (in 2007) Oyster mortality Australian autumn) microscopy Parasites present in connective Histology, tissue imprint, Haplosporidium tissue (mantle, gonads, digestive Protozoan Crassostrea virginica USA, Canada Spring-summer PCR, ISH, electron Unknown costale gland) but not in digestive tubule microscopy epithelia. Mortality in May-June Parasites present in gills, connective tissue. Sporulation only Histology, tissue imprint, Unknown but Haplosporidium Crassostrea virginica, USA, Canada, Japan, Korea, Summer (May until Protozoan in the epithelium of digestive gland smear, PCR, HIS, electron intermediate host is nelsoni Crassostrea gigas France October) in C. virginica, Mortalities in C. microscopy suspected virginica. No mortality in C. gigas Parasite present in connective Haplosporidium Protozoan O. edulis, O. angasi tissue. Sometimes, mortality can France, Netherland, Spain Histology, tissue imprint Unknown armoricanum be observed Ostrea edulis , Tiostrea Spring-summer Histology, tissue imprint, ISH, Intermediate host: Extracellular parasite of the Marteilia refringens Protozoan chilensis, O. -

Structure of Protistan Parasites Found in Bivalve Molluscs
W&M ScholarWorks VIMS Books and Book Chapters Virginia Institute of Marine Science 1988 Structure of Protistan Parasites Found in Bivalve Molluscs Frank O. Perkins Follow this and additional works at: https://scholarworks.wm.edu/vimsbooks Part of the Marine Biology Commons, and the Parasitology Commons American Fisheries Society Special Publication 18:93- 111 , 1988 CC> Copyrighl by !he American Fisheries Sociely 1988 PARASITE MORPHOLOGY, STRATEGY, AND EVOLUTION Structure of Protistan Parasites Found in Bivalve Molluscs 1 FRANK 0. PERKINS Virginia In stitute of Marine Science. School of Marine Science, College of William and Mary Gloucester Point, Virginia 23062, USA Abstral'I.-The literature on the structure of protists parasitizing bivalve molluscs is reviewed, and previously unpubli shed observations of species of class Perkinsea, phylum Haplosporidia, and class Paramyxea are presented. Descriptions are given of the flagellar apparatus of Perkin.His marinus zoospores, the ultrastructure of Perkinsus sp. from the Baltic macoma Maconw balthica, and the development of haplosporosome-like bodies in Haplosporidium nelsoni. The possible origin of stem cells of Marreilia sydneyi from the inner two sporoplasms is discussed. New research efforts are suggested which could help elucidate the phylogenetic interrelationships and taxonomic positions of the various taxa and help in efforts to better understand life cycles of selected species. Studies of the structure of protistan parasites terization of the parasite species, to elucidation of found in bivalve moll uscs have been fruitful to the many parasite life cycles, and to knowledge of morphologist interested in comparative morphol- parasite metabolism. The latter, especially, is ogy, evolu tion, and taxonomy. -

Shellfish Reefs at Risk
SHELLFISH REEFS AT RISK A Global Analysis of Problems and Solutions Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang CONTENTS Acknowledgments ........................................................................................................................ 1 Executive Summary .................................................................................................................... 2 Introduction .................................................................................................................................. 6 Methods .................................................................................................................................... 10 Results ........................................................................................................................................ 14 Condition of Oyster Reefs Globally Across Bays and Ecoregions ............ 14 Regional Summaries of the Condition of Shellfish Reefs ............................ 15 Overview of Threats and Causes of Decline ................................................................ 28 Recommendations for Conservation, Restoration and Management ................ 30 Conclusions ............................................................................................................................ 36 References ............................................................................................................................. -

Status of Angelshark, Squatina Squatina (Elasmobranchii: Squatiniformes: Squatinidae) in the Sea of Marmara
ANNALES · Ser. hist. nat. · 24 · 2014 · 1 Short scientifi c article UDK 597.315.6:591.9(262.53) Received: 2014-05-08 STATUS OF ANGELSHARK, SQUATINA SQUATINA (ELASMOBRANCHII: SQUATINIFORMES: SQUATINIDAE) IN THE SEA OF MARMARA Hakan KABASAKAL & Özgür KABASAKAL Ichthyological Research Society, Tantavi Mahallesi, Mentesoglu Caddesi, Idil Apt., No: 30, D: 4, Umraniye, TR-34764 Istanbul, Turkey E-mail: [email protected] ABSTRACT On 4 January 2014, a female specimen of Squatina squatina was entangled in trammel-net, at a depth of about 50 m. The specimen was 174 cm long (total length) and weighed approximately 35 kg. The recent single capture of S. squatina in the southeastern Sea of Marmara confi rms the contemporary presence of the species in this land- locked small marine region; however, the paucity of the species in the fi shing records of Marmaric fi shes since 2000, confi rms its rarity in the studied marine area. Keywords: Angelshark, Squatina squatina, Sea of Marmara, status, endangered, protection STATO DELL’ANGELO DI MARE, SQUATINA SQUATINA (ELASMOBRANCHII: SQUATINIFORMES: SQUATINIDAE), NEL MARE DI MARMARA SINTESI Il 4 gennaio 2014, una femmina di Squatina squatina è rimasta impigliata in una rete tramaglio, ad una profondità di circa 50 metri. La lunghezza totale dell’esemplare era pari a 174 cm per circa 35 kg di peso. La recente singola cattura di S. squatina nella parte sud-orientale del mare di Marmara conferma la presenza temporanea della specie in questa piccola semichiusa regione marina. Tuttavia, la scarsità di segnalazioni della specie nei registri di cattura della fauna ittica del mare di Marmara dal 2000, conferma la sua rarità nell’area marina studiata. -

Estudos Em Megabalanus Azoricus (Pilsbry, 1916)
Contributo para o conhecimento da biodiversidade regional – estudos em Megabalanus azoricus (Pilsbry, 1916). Dionísio, M.1, A. Pagarete2 & Costa, A.3 1, 2, 3 CIBIO – Centro de Investigação em Biodiversidade e Recursos Genéticos, Pólo Açores, Portugal; 2 – Departamento de Biologia, Universidade dos Açores, Rua da Mãe de Deus Apartado 1422, 9501-855 Ponta Delgada, Açores, Portugal. Tel. +351 296650100; Fax: +351 296650101 e-mail: 1 [email protected]; 2 [email protected]; 3 [email protected] RESUMO: Megabalanus azoricus (Pilsbry, 1916) é uma espécie nativa do arquipélago que apesar de ser reconhecida em toda a região onde sofre uma elevada exploração, só agora foi objecto de um estudo alargado. Apresentam-se aqui os primeiros resultados do projecto POCI/MAR/58185/2004 financiado pela FCT que visa o estudo dos aspectos da biologia desta espécie considerada pela OSPAR em risco e com necessidade de estudo urgente. A descrição de Megabalanus azoricus foi revista e pela primeira vez obtiveram-se larvas em laboratório. Iniciou-se uma inventariação de populações em várias ilhas do Arquipélago. Os estudos de reprodução realizados, baseados no estudo macroscópico e estereológico das gónadas de uma população de São Miguel permitiram verificar que estas cracas nos Açores apresentam-se sexualmente maturas ao longo de todo o ano, embora não se tenha registado produção de ovos em Novembro, Março e Agosto. Foram comparadas algumas populações provenientes de várias ilhas através da comparação das respectivas sequências de DNA de dois fragmentos COI-COII e 18S- ITS1 tendo-se verificado uma baixa variabilidade nos haplotipos de ambos os marcadores, o que não suporta níveis de diferenciação significativos entre as populações. -

Olympia Oyster (Ostrea Lurida)
COSEWIC Assessment and Status Report on the Olympia Oyster Ostrea lurida in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olympia Oyster Ostrea lurida in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 56 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 30 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Gillespie, G.E. 2000. COSEWIC status report on the Olympia Oyster Ostrea conchaphila in Canada in COSEWIC assessment and update status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-30 pp. Production note: COSEWIC acknowledges Graham E. Gillespie for writing the provisional status report on the Olympia Oyster, Ostrea lurida, prepared under contract with Environment Canada and Fisheries and Oceans Canada. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status reports were overseen by Robert Forsyth and Dr. Gerald Mackie, COSEWIC Molluscs Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l’huître plate du Pacifique (Ostrea lurida) au Canada. -

Angelshark (Squatina Squatina)
12th Meeting of the Conference of the Parties (CoP12) to the Convention on Migratory Species (CMS) Angelshark (Squatina squatina) Proposed action: Inclusion on CMS Appendices I and II Proponent: Monaco Overview Shark species around the world face a wide variety of threats, including being caught as bycatch. The overexploitation of the Angelshark, Squatina squatina for its meat, skin, and liver has made studying the species very difficult. The species’ seasonal north-south and onshore-offshore migrations are poorly documented largely because of the species’ scarcity. The available data shows the population decrease has caused some local or regional extinctions over most of its range, resulting in Squatina squatina being listed as Critically Endangered on the IUCN Red List. The decrease is largely due to targeted fisheries and, more recently, being caught incidentally as bycatch. The species is only adequately protected in some parts of its range and enforcement is lacking, which is why it is necessary to list the species on Appendices I and II. 12th Meeting of the Conference of the Parties (CoP12) to the Convention on Migratory Species (CMS) Biology and Distribution range is subject to intense demersal fisheries. This species is highly vulnerable from birth onwards to bycatch in the Squatina squatina is nocturnal, swimming at night and benthic trawls, set nets and bottom longlines which usually lying buried in sediment by day with only its eyes operate through most of its range and habitat. and dorsal fins protruding. It is an ambush predator that feeds primarily on bony fishes, cephalopods, skates, Uses crustaceans and mollusks. The species is a high level trophic predator. -

Eastern Atlantic and Mediterranean Angel Shark Conservation Strategy
© Carlos Suarez, Oceanos de Fuego Oceanos © Carlos Suarez, Squatina squatina Eastern Atlantic and Mediterranean Angel Shark Conservation Strategy Angelshark Sawback Angelshark Smoothback Angelshark Squatina squatina Squatina aculeata Squatina oculata Species background About this Strategy Angel sharks* rank as the second most threatened This Angel Shark Conservation Strategy provides a family of elasmobranch (sharks, skates and rays) after framework for improved protection of the three Critically sawfishes1. Characteristics linking the two families Endangered species present in the Eastern Atlantic and include their body shape and preferred habitat, as both Mediterranean. The Strategy aims to: improve the overall are large, flat-bodied coastal species. profile of angel sharks; increase the number of sightings reported; generate a better understanding of current The family Squatinidae contains at least 23 species, half distribution; contribute to IUCN Red List re-assessments of which are listed as threatened (Critically Endangered, and identify new collaboration opportunities to increase Endangered or Vulnerable) on the IUCN Red List of conservation action. Threatened SpeciesTM. Most of the remaining species are either Data Deficient or Not Evaluated. The slow Some of the key threats to these species are outlined growth and demersal nature of angel sharks leaves within this Strategy. Three priority goals and associated them especially vulnerable to inshore fishing activities. headline objectives have been identified as crucial to Consequently, many species in this family have suffered achieving the vision that: Angel sharks in the Eastern steep population declines and now face a significant risk Atlantic and Mediterranean are restored to robust of extinction. populations and safeguarded throughout their range. Once found throughout the temperate waters of the The recommended next steps outlined in this document Northeast Atlantic, Mediterranean and Black Seas, angel act as guidelines for targeted conservation actions.