Intrinsic Vulnerability in the Global Fish Catch
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fishing and Illegal Trade of the Angelshark DNA Barcoding
Fisheries Research 206 (2018) 193–197 Contents lists available at ScienceDirect Fisheries Research journal homepage: www.elsevier.com/locate/fishres The fishing and illegal trade of the angelshark: DNA barcoding against T misleading identifications ⁎ Ingrid Vasconcellos Bunholia, Bruno Lopes da Silva Ferrettea,b, , Juliana Beltramin De Biasia,b, Carolina de Oliveira Magalhãesa,b, Matheus Marcos Rotundoc, Claudio Oliveirab, Fausto Forestib, Fernando Fernandes Mendonçaa a Laboratório de Genética Pesqueira e Conservação (GenPesC), Instituto do Mar (IMar), Universidade Federal de São Paulo (UNIFESP), Santos, SP, 11070-102, Brazil b Laboratório de Biologia e Genética de Peixes (LBGP), Instituto de Biociências de Botucatu (IBB), Universidade Estadual Paulista (UNESP), Botucatu, SP, 18618-689, Brazil c Acervo Zoológico da Universidade Santa Cecília (AZUSC), Universidade Santa Cecília (Unisanta), Santos, SP, 11045-907, Brazil ARTICLE INFO ABSTRACT Handled by J Viñas Morphological identification in the field can be extremely difficult considering fragmentation of species for trade Keywords: or high similarity between congeneric species. In this context, the shark group belonging to the genus Squatina is Conservation composed of three species distributed in the southern part of the western Atlantic. These three species are Endangered species classified in the IUCN Red List as endangered, and they are currently protected under Brazilian law, which Fishing monitoring prohibits fishing and trade. Molecular genetic tools are now used for practical taxonomic identification, parti- Forensic genetics cularly in cases where morphological observation is prevented, e.g., during fish processing. Consequently, DNA fi Mislabeling identi cation barcoding was used in the present study to track potential crimes against the landing and trade of endangered species along the São Paulo coastline, in particular Squatina guggenheim (n = 75) and S. -

FISH LIST WISH LIST: a Case for Updating the Canadian Government’S Guidance for Common Names on Seafood
FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood Authors: Christina Callegari, Scott Wallace, Sarah Foster and Liane Arness ISBN: 978-1-988424-60-6 © SeaChoice November 2020 TABLE OF CONTENTS GLOSSARY . 3 EXECUTIVE SUMMARY . 4 Findings . 5 Recommendations . 6 INTRODUCTION . 7 APPROACH . 8 Identification of Canadian-caught species . 9 Data processing . 9 REPORT STRUCTURE . 10 SECTION A: COMMON AND OVERLAPPING NAMES . 10 Introduction . 10 Methodology . 10 Results . 11 Snapper/rockfish/Pacific snapper/rosefish/redfish . 12 Sole/flounder . 14 Shrimp/prawn . 15 Shark/dogfish . 15 Why it matters . 15 Recommendations . 16 SECTION B: CANADIAN-CAUGHT SPECIES OF HIGHEST CONCERN . 17 Introduction . 17 Methodology . 18 Results . 20 Commonly mislabelled species . 20 Species with sustainability concerns . 21 Species linked to human health concerns . 23 Species listed under the U .S . Seafood Import Monitoring Program . 25 Combined impact assessment . 26 Why it matters . 28 Recommendations . 28 SECTION C: MISSING SPECIES, MISSING ENGLISH AND FRENCH COMMON NAMES AND GENUS-LEVEL ENTRIES . 31 Introduction . 31 Missing species and outdated scientific names . 31 Scientific names without English or French CFIA common names . 32 Genus-level entries . 33 Why it matters . 34 Recommendations . 34 CONCLUSION . 35 REFERENCES . 36 APPENDIX . 39 Appendix A . 39 Appendix B . 39 FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood 2 GLOSSARY The terms below are defined to aid in comprehension of this report. Common name — Although species are given a standard Scientific name — The taxonomic (Latin) name for a species. common name that is readily used by the scientific In nomenclature, every scientific name consists of two parts, community, industry has adopted other widely used names the genus and the specific epithet, which is used to identify for species sold in the marketplace. -

Pleuronectidae, Poecilopsettidae, Achiridae, Cynoglossidae
1536 Glyptocephalus cynoglossus (Linnaeus, 1758) Pleuronectidae Witch flounder Range: Both sides of North Atlantic Ocean; in the western North Atlantic from Strait of Belle Isle to Cape Hatteras Habitat: Moderately deep water (mostly 45–330 m), deepest in southern part of range; found on mud, muddy sand or clay substrates Spawning: May–Oct in Gulf of Maine; Apr–Oct on Georges Bank; Feb–Jul Meristic Characters in Middle Atlantic Bight Myomeres: 58–60 Vertebrae: 11–12+45–47=56–59 Eggs: – Pelagic, spherical Early eggs similar in size Dorsal fin rays: 97–117 – Diameter: 1.2–1.6 mm to those of Gadus morhua Anal fin rays: 86–102 – Chorion: smooth and Melanogrammus aeglefinus Pectoral fin rays: 9–13 – Yolk: homogeneous Pelvic fin rays: 6/6 – Oil globules: none Caudal fin rays: 20–24 (total) – Perivitelline space: narrow Larvae: – Hatching occurs at 4–6 mm; eyes unpigmented – Body long, thin and transparent; preanus length (<33% TL) shorter than in Hippoglossoides or Hippoglossus – Head length increases from 13% SL at 6 mm to 22% SL at 42 mm – Body depth increases from 9% SL at 6 mm to 30% SL at 42 mm – Preopercle spines: 3–4 occur on posterior edge, 5–6 on lateral ridge at about 16 mm, increase to 17–19 spines – Flexion occurs at 14–20 mm; transformation occurs at 22–35 mm (sometimes delayed to larger sizes) – Sequence of fin ray formation: C, D, A – P2 – P1 – Pigment intensifies with development: 6 bands on body and fins, 3 major, 3 minor (see table below) Glyptocephalus cynoglossus Hippoglossoides platessoides Total myomeres 58–60 44–47 Preanus length <33%TL >35%TL Postanal pigment bars 3 major, 3 minor 3 with light scattering between Finfold pigment Bars extend onto finfold None Flexion size 14–20 mm 9–19 mm Ventral pigment Scattering anterior to anus Line from anus to isthmus Early Juvenile: Occurs in nursery habitats on continental slope E. -

Schedule Onlinepdf
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

DYNAMIC HABITAT USE of ALBACORE and THEIR PRIMARY PREY SPECIES in the CALIFORNIA CURRENT SYSTEM Calcofi Rep., Vol
MUHLING ET AL.: DYNAMIC HABITAT USE OF ALBACORE AND THEIR PRIMARY PREY SPECIES IN THE CALIFORNIA CURRENT SYSTEM CalCOFI Rep., Vol. 60, 2019 DYNAMIC HABITAT USE OF ALBACORE AND THEIR PRIMARY PREY SPECIES IN THE CALIFORNIA CURRENT SYSTEM BARBARA MUHLING, STEPHANIE BRODIE, MICHAEL JACOX OWYN SNODGRASS, DESIREE TOMMASI NOAA Earth System Research Laboratory University of California, Santa Cruz Boulder, CO Institute for Marine Science Santa Cruz, CA CHRISTOPHER A. EDWARDS ph: (858) 546-7197 Ocean Sciences Department [email protected] University of California, Santa Cruz, CA BARBARA MUHLING, OWYN SNODGRASS, YI XU HEIDI DEWAR, DESIREE TOMMASI, JOHN CHILDERS Department of Fisheries and Oceans NOAA Southwest Fisheries Science Center Delta, British Columbia, Canada San Diego, CA STEPHANIE SNYDER STEPHANIE BRODIE, MICHAEL JACOX Thomas More University, NOAA Southwest Fisheries Science Center Crestview Hills, KY Monterey, CA ABSTRACT peiods, krill, and some cephalopods (Smith et al. 2011). Juvenile north Pacific albacore Thunnus( alalunga) for- Many of these forage species are fished commercially, age in the California Current System (CCS), supporting but also support higher-order predators further up the fisheries between Baja California and British Columbia. food chain, such as other exploited species (e.g., tunas, Within the CCS, their distribution, abundance, and for- billfish) and protected resources (e.g., marine mammals aging behaviors are strongly variable interannually. Here, and seabirds) (Pikitch et al. 2004; Link and Browman we use catch logbook data and trawl survey records to 2014). Effectively managing marine ecosystems to pre- investigate how juvenile albacore in the CCS use their serve these trophic linkages, and improve robustness of oceanographic environment, and how their distributions management strategies to environmental variability, thus overlap with the habitats of four key forage species. -

Review of the Fishery Status for Whaler Sharks (Carcharhinus Spp.) in South Australian and Adjacent Waters
Review of the Fishery Status for Whaler Sharks (Carcharhinus spp.) in South Australian and adjacent waters Keith Jones FRDC Project Number 2004/067 January 2008 SARDI Aquatic Sciences Publication No. F2007/000721-1 SARDI Research Series No. 154 1 Review of the fishery status for whaler sharks in South Australian and adjacent waters. Final report to the Fisheries Research and Development Corporation. By: G.Keith Jones South Australian Research & Development Institute 2 Hamra Ave, West Beach SA 5022 (Current Address: PIRSA (Fisheries Policy) GPO Box 1625 Adelaide, SA 5001. Telephone: 08 82260439 Facsimile: 08 82262434 http://www.pirsa.saugov.sa.gov.au DISCLAIMER The author warrants that he has taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Chief Scientist. Although all reasonable efforts have been made to ensure quality, SARDI Aquatic Sciences does not warrant that the information in this report is free from errors or omissions. SARDI does not accept any liability for the contents of this report or for any consequences arising from its use or any other reliance placed upon it. © Copyright Fisheries Research and Development Corporation and South Australian Research & Development Institute, 2005.This work is copyright. Except as permitted under the Copyright Act 1968 (Commonwealth), no part of this publication may be reproduced by any process, electronic or otherwise, without the specific permission of the copyright owners. Neither may information be stored electronically in any form whatsoever without such permission. The Fisheries Research and Development Corporation plans, invests in and manages fisheries research and development throughout Australia. -

And Wildlife, 1928-72
Bibliography of Research Publications of the U.S. Bureau of Sport Fisheries and Wildlife, 1928-72 UNITED STATES DEPARTMENT OF THE INTERIOR BUREAU OF SPORT FISHERIES AND WILDLIFE RESOURCE PUBLICATION 120 BIBLIOGRAPHY OF RESEARCH PUBLICATIONS OF THE U.S. BUREAU OF SPORT FISHERIES AND WILDLIFE, 1928-72 Edited by Paul H. Eschmeyer, Division of Fishery Research Van T. Harris, Division of Wildlife Research Resource Publication 120 Published by the Bureau of Sport Fisheries and Wildlife Washington, B.C. 1974 Library of Congress Cataloging in Publication Data Eschmeyer, Paul Henry, 1916 Bibliography of research publications of the U.S. Bureau of Sport Fisheries and Wildlife, 1928-72. (Bureau of Sport Fisheries and Wildlife. Kesource publication 120) Supt. of Docs. no.: 1.49.66:120 1. Fishes Bibliography. 2. Game and game-birds Bibliography. 3. Fish-culture Bibliography. 4. Fishery management Bibliogra phy. 5. Wildlife management Bibliography. I. Harris, Van Thomas, 1915- joint author. II. United States. Bureau of Sport Fisheries and Wildlife. III. Title. IV. Series: United States Bureau of Sport Fisheries and Wildlife. Resource publication 120. S914.A3 no. 120 [Z7996.F5] 639'.9'08s [016.639*9] 74-8411 For sale by the Superintendent of Documents, U.S. Government Printing OfTie Washington, D.C. Price $2.30 Stock Number 2410-00366 BIBLIOGRAPHY OF RESEARCH PUBLICATIONS OF THE U.S. BUREAU OF SPORT FISHERIES AND WILDLIFE, 1928-72 INTRODUCTION This bibliography comprises publications in fishery and wildlife research au thored or coauthored by research scientists of the Bureau of Sport Fisheries and Wildlife and certain predecessor agencies. Separate lists, arranged alphabetically by author, are given for each of 17 fishery research and 6 wildlife research labora tories, stations, investigations, or centers. -
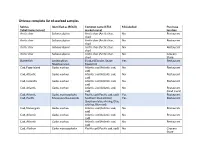
Ottawa: Complete List of Seafood Samples
Ottawa: complete list of seafood samples Sold as Identified as (BOLD) Common name (CFIA Mislabelled Purchase (label/menu/server) market name) location Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Grocery char) Store Butterfish Lepidocybium Escolar (Escolar, Snake Yes Restaurant flavobrunneum Mackerel) Cod, Fogo Island Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) (food truck) Cod, Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod, Pacific Micromesistius australis Southern blue whiting Yes Restaurant (Southern blue whiting, Blue whiting, Blue cod) Cod, Norwegian Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Alaskan Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Pacific Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, North Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Euro Bass Gadus morhua Atlantic cod (Atlantic cod, Yes Restaurant cod) Grouper Epinephelus diacanthus Spinycheek grouper (n/a) Yes – E. -

Congolli (Pseudaphritis Urvillii) and Australian Salmon (Arripis Truttaceus and A
Inland Waters and Catchment Ecology Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong George Giatas and Qifeng Ye SARDI Publication No. F2015/000479-1 SARDI Research Report Series No. 858 SARDI Aquatics Sciences PO Box 120 Henley Beach SA 5022 September 2015 Giatas and Ye (2015) Diet of three fish species in the Coorong Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong George Giatas and Qifeng Ye SARDI Publication No. F2015/000479-1 SARDI Research Report Series No. 858 September 2015 II Giatas and Ye (2015) Diet of three fish species in the Coorong This publication may be cited as: Giatas, G.C. and Ye, Q. (2015). Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2015/000479-1. SARDI Research Report Series No. 858. 81pp. South Australian Research and Development Institute SARDI Aquatic Sciences 2 Hamra Avenue West Beach SA 5024 Telephone: (08) 8207 5400 Facsimile: (08) 8207 5406 http://www.pir.sa.gov.au/research DISCLAIMER The authors warrant that they have taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Research Chief, Aquatic Sciences. Although all reasonable efforts have been made to ensure quality, SARDI does not warrant that the information in this report is free from errors or omissions. -

Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries
JANUARY 2020 Wholesale Market Profiles for Alaska Groundfish and FisheriesCrab Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries JANUARY 2020 JANUARY Prepared by: McDowell Group Authors and Contributions: From NOAA-NMFS’ Alaska Fisheries Science Center: Ben Fissel (PI, project oversight, project design, and editor), Brian Garber-Yonts (editor). From McDowell Group, Inc.: Jim Calvin (project oversight and editor), Dan Lesh (lead author/ analyst), Garrett Evridge (author/analyst) , Joe Jacobson (author/analyst), Paul Strickler (author/analyst). From Pacific States Marine Fisheries Commission: Bob Ryznar (project oversight and sub-contractor management), Jean Lee (data compilation and analysis) This report was produced and funded by the NOAA-NMFS’ Alaska Fisheries Science Center. Funding was awarded through a competitive contract to the Pacific States Marine Fisheries Commission and McDowell Group, Inc. The analysis was conducted during the winter of 2018 and spring of 2019, based primarily on 2017 harvest and market data. A final review by staff from NOAA-NMFS’ Alaska Fisheries Science Center was completed in June 2019 and the document was finalized in March 2016. Data throughout the report was compiled in November 2018. Revisions to source data after this time may not be reflect in this report. Typically, revisions to economic fisheries data are not substantial and data presented here accurately reflects the trends in the analyzed markets. For data sourced from NMFS and AKFIN the reader should refer to the Economic Status Report of the Groundfish Fisheries Off Alaska, 2017 (https://www.fisheries.noaa.gov/resource/data/2017-economic-status-groundfish-fisheries-alaska) and Economic Status Report of the BSAI King and Tanner Crab Fisheries Off Alaska, 2018 (https://www.fisheries.noaa. -

Polydactylus Opercularis (Gill, 1863) Fig
Threadfins of the World 65 Literature: Feltes in Carpenter (2003). Remarks: Although Polydactylus oligodon, originally described from Rio de Janeiro, Brazil and Jamaica on the basis of 2 specimens, had long been regarded as a junior synonym of P. virginicus by many authors, Randall (1966) recognized the former as valid and designated a lectotype for the species. Randall (1966) noted differences between P. oligodon and P. virginicus in the shape of the posterior margin of the maxilla, and certain meristic characters (including numbers of lateral-line scales, pectoral-fin rays and anal-fin rays) and proportional measurements (including length of anal-fin base). In particular, the rounded shape of the posterior margin of the maxilla has been subsequently treated as a diagnostic character for P. oligodon (versus truncate to concave in P. virginicus) in many publications (e.g. Randall in Fischer, 1978; Cervigón in Cervigón et al., 1993; Randall, 1996). According to Feltes in Carpenter (2003) and confirmed by examination by the author, however, that character shows considerable individual variation and it is difficult to clearly distinguish between P. oligodon and P. virginicus on that basis. Although P. oligodon and P. virginicus are very similar to each other and distinction between the 2 species in recent literature is somewhat confused, P. oligodon can be distinguished from the latter by the higher counts of pored lateral-line scales [67 to 73 (mode 70) versus 54 to 63 (mode 58) in the latter]. Polydactylus opercularis (Gill, 1863) Fig. 112; Plate IVb Trichidion opercularis Gill, 1863: 168 (type locality: west coast of Central America, probably Cape San Lucas, Baja California, Mexico; holotype apparently lost, see Motomura, Kimura and Iwatsuki, 2002). -

Catch Composition of the Western Australian Temperate Demersal Gillnet and Demersal Longline Fisheries, 1994 to 1999
FISHERIES RESEARCH REPORT NO. 146, 2003 Catch composition of the Western Australian temperate demersal gillnet and demersal longline fisheries, 1994 to 1999 R. McAuley and C. Simpfendorfer Fisheries Research Division WA Marine Research Laboratories PO Box 20 NORTH BEACH Western Australia 6920 Fisheries Research Report Titles in the fisheries research series contain technical and scientific information that represents an important contribution to existing knowledge, but which may not be suitable for publication in national or international scientific journals. Fisheries Research Reports may be cited as full publications. The full citation is: McAuley, R. and Simpfendorfer C. 2003. Catch composition of the Western Australian temperate demersal gillnet and demersal longline fisheries, 1994 to 1999, Fisheries Research Report No. 146, Department of Fisheries, Western Australia, 78 pp. Numbers 1-80 in this series were issued as Reports. Numbers 81-82 were issued as Fisheries Reports, and from number 83 the series has been issued under the current title. Enquiries Department of Fisheries 3rd floor The Atrium 168-170 St George’s Terrace PERTH WA 6000 Telephone (08) 9482 7333 Facsimile (08) 9482 7389 Website: http://www.fish.wa.gov.au/res Published by Department of Fisheries, Perth, Western Australia. November 2003. ISSN: 1035 - 4549 ISBN: 1 877098 36 1 An electronic copy of this report will be available at the above website where parts may be shown in colour where this is thought to improve clarity. Fisheries Research in Western Australia The Fisheries Research Division of the Department of Fisheries is based at the Western Australian Marine Research Laboratories, PO Box 20, North Beach (Perth), Western Australia, 6920.