The Olympia Oyster Ostrea Lurida: Recent Advances in Natural History
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The History and Decline of Ostrea Lurida in Willapa Bay, Washington Author(S): Brady Blake and Philine S
The History and Decline of Ostrea Lurida in Willapa Bay, Washington Author(s): Brady Blake and Philine S. E. Zu Ermgassen Source: Journal of Shellfish Research, 34(2):273-280. Published By: National Shellfisheries Association DOI: http://dx.doi.org/10.2983/035.034.0208 URL: http://www.bioone.org/doi/full/10.2983/035.034.0208 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Shellfish Research, Vol. 34, No. 2, 273–280, 2015. THE HISTORY AND DECLINE OF OSTREA LURIDA IN WILLAPA BAY, WASHINGTON BRADY BLAKE1 AND PHILINE S. E. ZU ERMGASSEN2* 1Washington State Department of Fish and Wildlife, 375 Hudson Street, Port Townsend, WA 98368; 2Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, United Kingdom ABSTRACT With an annual production of 1500 metric tons of shucked oysters, Willapa Bay, WA currently produces more oysters than any other estuary in the United States. -

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -
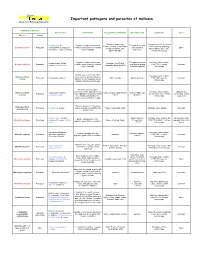
Pathogens Table 2006
Important pathogens and parasites of molluscs Genetic and Pathology Laboratory Pathogen or parasite Host species Host impact Geographical distribution Infection period Diagnostic Cycle Species Group Denmark, Netherland, Incubation period : 3-4 month Ostrea edulis, O. Parasite of oyster haemocytes Throughout the year France, Ireland, Great Britain in infected area. Histology, Bonamia ostreae Protozoan Conchaphila, O. Angasi, O. (=>all the tissues can be invaded). (with a peak in Direct (except Scotland), Italy, tissue imprint, PCR, ISH, Puelchana, Tiostrea chilensis Oyster mortality September) Spain and USA electron microscopy Parasite of oyster haemocytes Throughout the year Histology, tissue imprint, Ostrea angasi, Ostrea Australia, New Zéland, Bonamia exitiosa Protozoan (=>all the tissues can be invaded). (with a peak during PCR, ISH, electron Unknown chilensis, Ostrea edulis Tasmania, Spain (in 2007) Oyster mortality Australian autumn) microscopy Parasites present in connective Histology, tissue imprint, Haplosporidium tissue (mantle, gonads, digestive Protozoan Crassostrea virginica USA, Canada Spring-summer PCR, ISH, electron Unknown costale gland) but not in digestive tubule microscopy epithelia. Mortality in May-June Parasites present in gills, connective tissue. Sporulation only Histology, tissue imprint, Unknown but Haplosporidium Crassostrea virginica, USA, Canada, Japan, Korea, Summer (May until Protozoan in the epithelium of digestive gland smear, PCR, HIS, electron intermediate host is nelsoni Crassostrea gigas France October) in C. virginica, Mortalities in C. microscopy suspected virginica. No mortality in C. gigas Parasite present in connective Haplosporidium Protozoan O. edulis, O. angasi tissue. Sometimes, mortality can France, Netherland, Spain Histology, tissue imprint Unknown armoricanum be observed Ostrea edulis , Tiostrea Spring-summer Histology, tissue imprint, ISH, Intermediate host: Extracellular parasite of the Marteilia refringens Protozoan chilensis, O. -

Structure of Protistan Parasites Found in Bivalve Molluscs
W&M ScholarWorks VIMS Books and Book Chapters Virginia Institute of Marine Science 1988 Structure of Protistan Parasites Found in Bivalve Molluscs Frank O. Perkins Follow this and additional works at: https://scholarworks.wm.edu/vimsbooks Part of the Marine Biology Commons, and the Parasitology Commons American Fisheries Society Special Publication 18:93- 111 , 1988 CC> Copyrighl by !he American Fisheries Sociely 1988 PARASITE MORPHOLOGY, STRATEGY, AND EVOLUTION Structure of Protistan Parasites Found in Bivalve Molluscs 1 FRANK 0. PERKINS Virginia In stitute of Marine Science. School of Marine Science, College of William and Mary Gloucester Point, Virginia 23062, USA Abstral'I.-The literature on the structure of protists parasitizing bivalve molluscs is reviewed, and previously unpubli shed observations of species of class Perkinsea, phylum Haplosporidia, and class Paramyxea are presented. Descriptions are given of the flagellar apparatus of Perkin.His marinus zoospores, the ultrastructure of Perkinsus sp. from the Baltic macoma Maconw balthica, and the development of haplosporosome-like bodies in Haplosporidium nelsoni. The possible origin of stem cells of Marreilia sydneyi from the inner two sporoplasms is discussed. New research efforts are suggested which could help elucidate the phylogenetic interrelationships and taxonomic positions of the various taxa and help in efforts to better understand life cycles of selected species. Studies of the structure of protistan parasites terization of the parasite species, to elucidation of found in bivalve moll uscs have been fruitful to the many parasite life cycles, and to knowledge of morphologist interested in comparative morphol- parasite metabolism. The latter, especially, is ogy, evolu tion, and taxonomy. -

Shellfish Reefs at Risk
SHELLFISH REEFS AT RISK A Global Analysis of Problems and Solutions Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang CONTENTS Acknowledgments ........................................................................................................................ 1 Executive Summary .................................................................................................................... 2 Introduction .................................................................................................................................. 6 Methods .................................................................................................................................... 10 Results ........................................................................................................................................ 14 Condition of Oyster Reefs Globally Across Bays and Ecoregions ............ 14 Regional Summaries of the Condition of Shellfish Reefs ............................ 15 Overview of Threats and Causes of Decline ................................................................ 28 Recommendations for Conservation, Restoration and Management ................ 30 Conclusions ............................................................................................................................ 36 References ............................................................................................................................. -

Environmental History and Oysters at Point Reyes National Seashore
Restoring the Past: Environmental History and Oysters at Point Reyes National Seashore Timothy Babalis Since its inception more than 40 years ago, environmental history has matured into a respected, if somewhat nebulous, discipline in academic circles but has so far received less attention within public land management agencies such as the National Park Service.1 This is unfortunate, because environmental history can provide information of great practical interest to resource managers as well as offering a valuable perspective on management prac- tices. The singular characteristic which distinguishes environmental history from other his- torical methodologies is the acknowledgement that history happens in places. Like geogra- phers, whose field is closely related, environmental historians consider the spatial dimension of history to be just as important as its temporal. As a result, the physical environment is one of environmental history’s principal subjects, along with the usual human actors, political events, and cultural expressions of traditional history. But environmental history also acknowledges the active capacity of the environment to influence and form human history,as well as being the place where that history unfolds. Environmental historians study the recip- rocal relationship between human societies and the physical environments they inhabit. As one prominent environmental historian has written, “When I use the term ‘environmental history,’I mean specifically the history of the consequences of human actions on the environ- ment and the reciprocal consequences of an altered nature for human society.”2 While most environmental historians agree on this basic formula, the field quickly diverges in a bewildering number of directions and becomes increasingly difficult to catego- rize or define. -

Olympia Oyster (Ostrea Lurida)
COSEWIC Assessment and Status Report on the Olympia Oyster Ostrea lurida in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olympia Oyster Ostrea lurida in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 56 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 30 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Gillespie, G.E. 2000. COSEWIC status report on the Olympia Oyster Ostrea conchaphila in Canada in COSEWIC assessment and update status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-30 pp. Production note: COSEWIC acknowledges Graham E. Gillespie for writing the provisional status report on the Olympia Oyster, Ostrea lurida, prepared under contract with Environment Canada and Fisheries and Oceans Canada. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status reports were overseen by Robert Forsyth and Dr. Gerald Mackie, COSEWIC Molluscs Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l’huître plate du Pacifique (Ostrea lurida) au Canada. -

Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi Alvar Carranza, Loren D
Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, M atthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang Results. Condition of Oyster Reefs Globally Across Bays and Ecoregions. Regional Summaries of the Condition of Shellfish Reefs Overview of Threats and Causes of Decline. Recommendations for Conservation, Restoration and Management Conclusions References Appendix 1 Michael W. Beck“, Robert D. Brumbaughb, Laura AiroldL, Alvar Carranzad, Loren D. Coen*, Christine Crawfordi Omar Defeod, Graham J. Edgarf, Boze Hancock®, Matthew Kayh, Hunter Lenihan11, Mark W. Luckenbach', Caitlyn L. Toropova“, Guofan Zhang “ The Nature Conservancy, Institute of Marine Sciences, University of California, Santa Cruz, CA, 95060 b b The Nature Conservancy; PO Box 420237, Summerland Key, FL 33042 * Dipartimento di Biología Evoluziomstica Sperimentale, Université di Bologna, Via S. Alberto 163,1-48100 Ravenna, Italy d d Marine Science Unit, Ecology Department, Faculty of Sciences, Montevideo, Uruguay * Sanibel-Captiva Conservation Foundation, 9 0 0 A Tarpon Bay Road, Sanibel, FL 33957 f Tasmanian Aquaculture and Fisheries Institute, University of Tasmania, Hobart, Tasmania, Australia ® The Nature Conservancy; University of Rhode Island, Narragansett, Rl 028882 h Bren School, University of California, Santa Barbara, CA 93106-5131 ' Virginia Institute of Marine Science, College of William and Mary, Wachapreague, VA 23480 i Institute of Oceanology, Chinese Academy of Sciences, Qingdao, Shandong 266071, China Cover photo: Oyster reets at Virginia Coastal Reserve. © Barry T ru itt/T N C © Barry T ru itt/T N C Many colleagues contributed to this assessment by The authors in particular thank Christine Shepard, Zach providing access to data sets ranging from local to global Ferdaña, Jeff Vincent, Antonella Fatone, Ximing Guo, and scales, helping to find important and often obscure Bill Arnold for help with the data, figures and maps. -

From British Columbia to Baja California Restoring the Olympia Oyster (Ostrea Lurida)
From British Columbia to Baja California Restoring The Olympia Oyster (Ostrea lurida) Report of a Forum Sponsored by American Honda Motor Corporation Aquarium of the Pacific Bren School of the University of California, Santa Barbara 16-17 March 2017 Acknowledgements We thank the Bren Oyster Group, all of the other presenters, and those who authored sections of this report. We thank the Bren students for acting as rapporteurs and for their excellent notes. We also thank Linda Brown for handling the logistics from start to finish and for helping assemble the sections of this report. We also thank Linda Brown and Claire Atkinson for editing the report. Jerry R. Schubel, Aquarium of the Pacific Steven Center, American Honda Motor Co., Inc. Hunter Lenihan, UC Santa Barbara This report can be found at: http://www.aquariumofpacific.org/mcri/info/restoring_the_olympia_oyster/forums 2 Table of Contents Introduction .......................................................................................................................... 5 Insights from the forum ......................................................................................................... 9 Action Items ....................................................................................................................... 11 Planning and Incentivizing Native Olympia Oyster Restoration in SoCal ........................... 13 Restoration of Native Oysters in San Francisco Bay .......................................................... 21 Restoration of Native Oysters in -

And Transfers
MOLLUSCAN INTRODUCTIONS AND TRANSFERS A Maryland Sea Grant Publication College Park Maryland MOLLUSCAN INTRODUCTIONS AND TRANSFERS MOLLUSCAN INTRODUCTIONS AND TRANSFERS Rrsx CoNSIDERATIONs AND IMPLICATIONS A Symposium Proceedings Edited by ] ames T. Carlton and Aaron Rosenfield ...,.~ . (.......-~j/4!1!!f~~ A Maryland Sea Grant Publication ·~ .. College Park, Maryland Published by the Maryland Sea Grant College, University of Maryland, College Park. Publication of this book is supported by grant #NA46RG009l from the National Oceanic and Atmospheric Administra tion to the Maryland Sea Grant College and by Grant #NA90AA-D-SG 184. The papers in this book were presented at a special symposium, Molluscan Introductions and Transfers: Risk Consider ations and Implications, presented at the 82nd Annual Meeting of the National Shellfisheries Association and the Shellfish Institute of North America, held April 4-5, 1990 in Williamsburg, Virginia. All the papers are reprinted with the permission of the Journal of Shellfish Research. Copyright © 1994 Maryland Sea Grant College. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, elec tronic or mechanical, including photocopying, recording, or any information storage or retrival system, without permis sion in writing from Maryland Sea Grant. Sea Grant is a federal-state-university partnership encouraging the wise stewardship of our marine resources through research, education and technology transfer. University of Maryland Publication UM-SG-TS-94-02 ISBN: 0-943676-58-4 For information on Maryland Sea Grant publications, contact: Maryland Sea Grant College 0112 Skinner Hall University of Maryland System College Park, Maryland 20742 Printed on recycled paper. -

United States National Museum
* si 'a*»/ ^ ^ l^// kh < (M->'^^'' ^eparfrrxenf of fhc inferior: U. S. NATIONAL MUSEUM. 21 BULLETIN UNITED STATES NATIONAL MUSEUM. NO. 18.—EXHIBIT OF THE FISHERIES AND FISH CULTURE OP THE ^^^ —-UNITED STATES OF AMERfCA. MADE AT BERLIN IN 1880. PREPARED UXDEU THE DIRECTION OF a. BROA^^ls^ ooode, DEPUTY COiTMISSIONEE. WASHINGTON: aOVFiRNMENT PETNTTNG- OFFICJE 18 80. '^epavimeni of ihc 55nfcrior U. a. NATIONAL MUSEUM. 21 BULLETIN unu'ei) states national museum. No. 18. PUBLISHED UNDER THE DIRECTION OF THE SMITHSONIAN INSTITUTION. WASHINGTON: GOVERNMENT PRINTING OFFICE. 1880. ADYEETISEMENT. This work is the twenty-first of a series of papers intended to illnstrate the collections of natural history and ethnology belonging- to the United States, and constituting the i^ational Museum, of which the Smithsonian Institution was placed in charge by the act of Congress of August 10, 1846. It has been prepared at the request of the Smithsonian Institution, and printed by authority of the honorable Secretary of the Interior. SPEXCER F. BAIRD, Secretary of the Snuthsonian Institution. Smithsonian Institution, Washington, March 29, 1880. INTERNATIONAL FISHERY EXHIBITION, BERLIN, 1880. EXHIBIT THE FISHERIES AND FISH CULTlIPiE UNITED STATES OF AMERICA, INTERNATIONALE FISCHEEEI-AUSSTELLUNG, HELD AT BERLIN, APRIL 20, 1880, AND FORMING A PART OF THE COL- LECTIONS OF THE NATIONAL MUSEmi, MADE BY THE UNITED STATES FISH COMMISSION. PKEPARED UNDEU THE DIRECTION OI' a. BIlo^^^]s^ aooDE, DEPUTY COMMISSIONEK. WASHINGTOIT: <3-OVEENMENT FEINTING OFPIOE. 1880. TABLE OF CONTENTS. Section A.—AQUATIC ANIMALS AND PLANTS OF NOKTH AMERICA BENE- FICIAL OR INJURIOUS TO MAN. VERTEBKATES. Page. I. Mammals 1 1. Ferae (carnivores) 1 Fissipedia (laud carnivores) 1 Piunipedia (seals, Sec. -

Is Pallial Mucus Involved in Ostrea Edulis Defenses Against the Parasite Bonamia
1 Is pallial mucus involved in Ostrea edulis defenses against the parasite Bonamia 2 ostreae? 3 Sergio Fernández-Boo1,4*, Ophélie Gervais1, Maria Prado-Alvarez3 Bruno Chollet1, 4 Stéphane Claverol2, Cyrielle Lecadet1, Christine Dubreuil1, Isabelle Arzul1 5 1 Institut Français de Recherche pour l´Exploitation de la Mer (IFREMER), Laboratoire de 6 Génétique et Pathologie (LGP), Avenue Mus de Loup, 17390 La Tremblade, France. 7 2 Université de Bordeaux, Centre Génomique Fonctionnelle de Bordeaux, Plateforme 8 Protéome, F-33000 Bordeaux, France. 9 3 Aquatic Molecular Pathobiology Group. Marine Research Institute (IIM-CSIC). Vigo. 10 Spain. 11 4 Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR), University of 12 Porto, Avenida General Norton de Matos, S/N, 4450-208 Matosinhos, Portugal. 13 14 *Corresponding author: [email protected] 15 Abstract 16 Bonamia ostreae is an intrahemocytic parasite that has been responsible for severe 17 mortalities in the flat oyster Ostrea edulis since the 1970´s. The Pacific oyster 18 Crassostrea gigas is considered to be resistant to the disease and appears to have 19 mechanisms to avoid infection. Most studies carried out on the invertebrate immune 20 system focus on the role of hemolymph, although mucus, which covers the body surface 21 of molluscs, could also act as a barrier against pathogens. In this study, the in vitro effect 22 of mucus from the oyster species Ostrea edulis and C. gigas on B. ostreae was 23 investigated using flow cytometry. Results showed an increase in esterase activities and 24 mortality rate of parasites exposed to mucus from both oyster species.