Article Is of Individuals at Depth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Otoliths in Situ in the Stem Teleost Cavenderichthys Talbragarensis
Journal of Vertebrate Paleontology ISSN: 0272-4634 (Print) 1937-2809 (Online) Journal homepage: https://www.tandfonline.com/loi/ujvp20 Otoliths in situ in the stem teleost Cavenderichthys talbragarensis (Woodward, 1895), otoliths in coprolites, and isolated otoliths from the Upper Jurassic of Talbragar, New South Wales, Australia Werner W. Schwarzhans, Timothy D. Murphy & Michael Frese To cite this article: Werner W. Schwarzhans, Timothy D. Murphy & Michael Frese (2018) Otoliths in situ in the stem teleost Cavenderichthystalbragarensis (Woodward, 1895), otoliths in coprolites, and isolated otoliths from the Upper Jurassic of Talbragar, New South Wales, Australia, Journal of Vertebrate Paleontology, 38:6, e1539740, DOI: 10.1080/02724634.2018.1539740 To link to this article: https://doi.org/10.1080/02724634.2018.1539740 © 2019 Werner W. Schwarzhans, Timothy View supplementary material D. Murphy, and Michael Frese. Published by Informa UK Limited, trading as Taylor & Francis Group. Published online: 19 Feb 2019. Submit your article to this journal Article views: 619 View related articles View Crossmark data Citing articles: 1 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ujvp20 Journal of Vertebrate Paleontology e1539740 (14 pages) © Published with license by the Society of Vertebrate Paleontology DOI: 10.1080/02724634.2018.1539740 ARTICLE OTOLITHS IN SITU IN THE STEM TELEOST CAVENDERICHTHYS TALBRAGARENSIS (WOODWARD, 1895), OTOLITHS IN COPROLITES, -

Response of Deep-Sea Scavengers to Ocean Acidification and the Odor from a Dead Grenadier
Vol. 350: 193–207, 2007 MARINE ECOLOGY PROGRESS SERIES Published November 22 doi: 10.3354/meps07188 Mar Ecol Prog Ser OPENPEN ACCESSCCESS Response of deep-sea scavengers to ocean acidification and the odor from a dead grenadier James P. Barry1,*, Jeffrey C. Drazen2 1Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 2University of Hawaii, Department of Oceanography, MSB 606, 1000 Pope Rd, Honolulu, Hawaii 96822, USA ABSTRACT: Experiments to assess the impact of ocean acidification on abyssal animals were per- formed off Central California. The survival of caged megafauna (Benthoctopus sp., Pachycara bulbi- ceps, Coryphaenoides armatus) exposed to CO2-rich and normal (control) seawater varied among species. Benthoctopus sp. and P. bulbiceps survived control conditions and month-long episodic exposure to acidic, CO2-rich waters (pH reductions of ~0.1 U). All C. armatus in both treatments died, potentially due to cage-related stress, predation, and exposure to acidic waters. High survival by P. bulbiceps and Benthoctopus under month-long exposure to CO2-rich waters indicates a physiologi- cal capacity to cope, at least temporarily, with stresses that will accompany expected future changes in ocean chemistry. The abundance of free-ranging scavengers was not correlated with variation in pH levels near fish cages. Incidental observations of abyssal scavengers collected using time-lapse cameras during these experiments were used secondarily to evaluate the hypothesis that macrourid fishes avoid the odor of dead conspecifics. Caged macrourids in view of time-lapse cameras died within 2 to 3 d, eliciting a strong response from the regional scavenger assemblage which aggregated near the cage. -
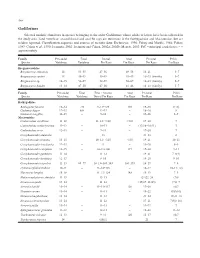
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

The Fish Fauna of Ampe`Re Seamount (NE Atlantic) and the Adjacent
Helgol Mar Res (2015) 69:13–23 DOI 10.1007/s10152-014-0413-4 ORIGINAL ARTICLE The fish fauna of Ampe`re Seamount (NE Atlantic) and the adjacent abyssal plain Bernd Christiansen • Rui P. Vieira • Sabine Christiansen • Anneke Denda • Frederico Oliveira • Jorge M. S. Gonc¸alves Received: 26 March 2014 / Revised: 15 September 2014 / Accepted: 24 September 2014 / Published online: 2 October 2014 Ó Springer-Verlag Berlin Heidelberg and AWI 2014 Abstract An inventory of benthic and benthopelagic stone’’ hypothesis of species dispersal, some differences fishes is presented as a result of two exploratory surveys can be attributed to the local features of the seamounts. around Ampe`re Seamount, between Madeira and the Por- tuguese mainland, covering water depths from 60 to Keywords Deep sea Á Fish distribution Á Ichthyofauna Á 4,400 m. A total of 239 fishes were collected using dif- Seamounts Á Zoogeography ferent types of sampling gear. Three chondrichthyan spe- cies and 31 teleosts in 21 families were identified. The collections showed a vertical zonation with little overlap, Introduction but indications for an affinity of species to certain water masses were only vague. Although most of the species Due to their vertical range and habitat diversity, seamounts present new records for Ampe`re Seamount, all of them often support high fish diversity, as compared to the sur- have been known for the NE Atlantic; endemic species rounding ocean, and some are known as hotspots of were not found. The comparison with fish communities at endemic species (e.g. Shank 2010; Stocks et al. 2012). other NE Atlantic seamounts indicates that despite a high Seamounts are considered to act as ‘‘stepping stones’’ for ichthyofaunal similarity, which supports the ‘‘stepping species dispersal (Almada et al. -

The Absence of Sharks from Abyssal Regions of the World's Oceans
Proc. R. Soc. B (2006) 273, 1435–1441 doi:10.1098/rspb.2005.3461 Published online 21 February 2006 The absence of sharks from abyssal regions of the world’s oceans Imants G. Priede1,*, Rainer Froese2, David M. Bailey3, Odd Aksel Bergstad4, Martin A. Collins5, Jan Erik Dyb6, Camila Henriques1, Emma G. Jones7 and Nicola King1 1University of Aberdeen, Oceanlab, Newburgh, Aberdeen AB41 6AA, UK 2Leibniz-Institut fu¨r Meereswissenschaften, IfM-GEOMAR, Du¨sternbrooker Weg 20, 24105 Kiel, Germany 3Marine Biology Research Division, Scripps Institution of Oceanography, UCSD 9500 Gilman Drive, La Jolla, CA 92093-0202, USA 4Institute of Marine Research, Flødevigen Marine Research Station, 4817 His, Norway 5British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 0ET, UK 6Møre Research, Section of Fisheries, PO Box 5075, 6021 Aalesund, Norway 7FRS Marine Laboratory, 375 Victoria Road, Aberdeen AB11 9DB, UK The oceanic abyss (depths greater than 3000 m), one of the largest environments on the planet, is characterized by absence of solar light, high pressures and remoteness from surface food supply necessitating special molecular, physiological, behavioural and ecological adaptations of organisms that live there. Sampling by trawl, baited hooks and cameras we show that the Chondrichthyes (sharks, rays and chimaeras) are absent from, or very rare in this region. Analysis of a global data set shows a trend of rapid disappearance of chondrichthyan species with depth when compared with bony fishes. Sharks, apparently well adapted to life at high pressures are conspicuous on slopes down to 2000 m including scavenging at food falls such as dead whales. -

DEEPFISHMAN Document 5 : Review of Parasites, Pathogens
DEEPFISHMAN Management And Monitoring Of Deep-sea Fisheries And Stocks Project number: 227390 Small or medium scale focused research action Topic: FP7-KBBE-2008-1-4-02 (Deepsea fisheries management) DEEPFISHMAN Document 5 Title: Review of parasites, pathogens and contaminants of deep sea fish with a focus on their role in population health and structure Due date: none Actual submission date: 10 June 2010 Start date of the project: April 1st, 2009 Duration : 36 months Organization Name of lead coordinator: Ifremer Dissemination Level: PU (Public) Date: 10 June 2010 Review of parasites, pathogens and contaminants of deep sea fish with a focus on their role in population health and structure. Matt Longshaw & Stephen Feist Cefas Weymouth Laboratory Barrack Road, The Nothe, Weymouth, Dorset DT4 8UB 1. Introduction This review provides a summary of the parasites, pathogens and contaminant related impacts on deep sea fish normally found at depths greater than about 200m There is a clear focus on worldwide commercial species but has an emphasis on records and reports from the north east Atlantic. In particular, the focus of species following discussion were as follows: deep-water squalid sharks (e.g. Centrophorus squamosus and Centroscymnus coelolepis), black scabbardfish (Aphanopus carbo) (except in ICES area IX – fielded by Portuguese), roundnose grenadier (Coryphaenoides rupestris), orange roughy (Hoplostethus atlanticus), blue ling (Molva dypterygia), torsk (Brosme brosme), greater silver smelt (Argentina silus), Greenland halibut (Reinhardtius hippoglossoides), deep-sea redfish (Sebastes mentella), alfonsino (Beryx spp.), red blackspot seabream (Pagellus bogaraveo). However, it should be noted that in some cases no disease or contaminant data exists for these species. -

Biodiversity of Bear Seamount, New England Seamount Chain: Results of Exploratory Trawling
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 9-2003 Biodiversity of Bear Seamount, New England Seamount chain: Results of exploratory trawling JA Moore M Vecchione R Gibbons JK Galbraith M Turnipseed Virginia Institute of Marine Science See next page for additional authors Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons, and the Marine Biology Commons Recommended Citation Moore, JA; Vecchione, M; Gibbons, R; Galbraith, JK; Turnipseed, M; Southworth, M; and Watkins, E, Biodiversity of Bear Seamount, New England Seamount chain: Results of exploratory trawling (2003). Journal of Northwest Atlantic Fishery Science, 31, 363-372. https://scholarworks.wm.edu/vimsarticles/1970 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Authors JA Moore, M Vecchione, R Gibbons, JK Galbraith, M Turnipseed, M Southworth, and E Watkins This article is available at W&M ScholarWorks: https://scholarworks.wm.edu/vimsarticles/1970 J Northw Atl Fish Sci, Vol 31: 363372 Biodiversity of Bear Seamount, New England Seamount Chain: Results of Exploratory Trawling J A Moore Florida Atlantic University, Honors College, Jupiter, FL 33458, USA M Vecchione, B B Collette and R Gibbons National Marine Fisheries Service, National Systematics Laboratory, -

DEEPFISH Project: Applying an Ecosystem Approach to the Sustainable Management of Deep-Water Fisheries
DEEPFISH Project: Applying an ecosystem approach to the sustainable management of deep-water fisheries. Part 1: Development of an Ecopath with Ecosim model SAMS report no. 259a DEEPFISH Project: Applying an ecosystem approach to the sustainable management of deep-water fisheries Project Partners University of Plymouth: Dr Kerry Howell Scottish Association for Marine Science: Dr Sheila JJ Heymans, Dr John Gordon, Janet Duncan, Morag Ayers1 Additional contributions from Fisheries Research Services: Dr Emma Jones2 1 Now at the School of Biological and Conservation Sciences, University of Kwazulu-Natal, 4041 Durban, South Africa. 2 Now at the National Institute of Water and Atmospheric Research, New Zealand 1 A report detailing the project findings is available (DEEPFISH Project: Part 2 report), for a copy please contact Dr Kerry Howell at the University of Plymouth, UK. French and Spanish versions of both reports are also available as pdf documents. The DEEPFISH project is a collaboration between the University of Plymouth and the Scottish Association for Marine Science with data contributions from Fisheries Research Services, Aberdeen (now Marine Scotland). It is funded by the Esmée Fairbairn Foundation and a RCUK Academic Fellowship awarded to Dr Howell. This report should be referenced as: Howell, K.L., Heymans, J.J., Gordon, J.D.M., Duncan, J., Ayers, M., Jones, E.G (2009) DEEPFISH Project: Applying an ecosystem approach to the sustainable management of deep-water fisheries. Part 1: Development of the Ecopath with Ecosim model. Scottish Association for Marine Science, Oban. U.K. Report no. 259a. Image credits: Images indicated as © Crown Copyright, all rights reserved. -

Small-Scale Patterns of Deep-Sea Fish Distributions and Assemblages Of
Deep-Sea Research I 65 (2012) 171–188 Contents lists available at SciVerse ScienceDirect Deep-Sea Research I journal homepage: www.elsevier.com/locate/dsri Small-scale patterns of deep-sea fish distributions and assemblages of the Grand Banks, Newfoundland continental slope Krista D. Baker a,n, Richard L. Haedrich a, Paul V.R. Snelgrove b, Vonda E. Wareham c, Evan N. Edinger d, Kent D. Gilkinson c a Biology Department, Memorial University of Newfoundland, St. John’s, NL, Canada A1B 3X9 b Ocean Sciences Centre and Biology Department, Memorial University of Newfoundland, St. John’s, NL, Canada A1C 5S7 c Fisheries and Oceans Canada, Northwest Atlantic Fisheries Centre, St. John’s, NL, Canada A1C 5X1 d Geography Department and Biology Department, Memorial University of Newfoundland, St. John’s, NL, Canada A1B 3X9 article info abstract Article history: Deep-sea fishes are the target of directed fisheries and are considered a conservation concern. Yet, we Received 20 September 2011 still know little about the factors that affect deep-sea fish distributions and assemblage patterns on Received in revised form relatively small spatial scales. We used results from remotely operated vehicle surveys that observed 19 March 2012 105 km (346 960 m2) of seafloor over a depth range of 351–2245 m in three canyons off Newfound- Accepted 28 March 2012 land to examine the occurrence, behavior, habitat specificity, and regional assemblage patterns of deep- Available online 5 April 2012 sea fishes in this region. We found distinct assemblages based on both depth and habitat classifications. Keywords: The most obvious unique assemblage was that associated with outcrops, which served as habitat for Fish assemblage patterns relatively rare species such as Neocyttus helgae, Hoplostethus atlanticus, and Lepidion eques. -

Energetics of Grenadier Fishes
American Fisheries Society Symposium 63:xxx, 2008 © 2008 by the American Fisheries Society Energetics of Grenadier Fishes JEFFREY C. DRAZEN† University of Hawaii, Department of Oceanography, MSB606 1000 Pope Road, Honolulu, Hawaii 96822, USA Abstract.—Energetic parameters such as metabolism, growth, and reproduction rep- resent investments by an animal in maintenance and production. The available lit- erature was reviewed to examine trends in these parameters for grenadiers to better understand their biology in relationship to shallow living species. Grenadiers are adapted to deep-sea habitats that have fundamentally different environmental condi- tions than the continental shelves where most exploited fishes live. Grenadiers have very low metabolic rates, similar to other deep-sea demersal and pelagic fishes. This appears to be the result of a relaxation in the selective pressure for locomotory capac- ity in dim or totally dark waters. Longevities are variable and dependant, in part, on body size. Regardless of longevity, low rates of mass-specific growth are typical and may be limited by its relationship to metabolism. Finally, reproductive outputs may be much lower than originally anticipated from gross measures of fecundity, and at least some species may reproduce less often than annually. Energetic data are sparse but until more are available for diverse species, we must assume that with a similar body form, phylogeny, and habitat, that all grenadiers have low rates of metabolism, growth, and reproduction. Grenadiers are exceptionally diverse and certainly varia- tion exists, some of which can be explained by individual ecologies. Nevertheless it is clear that models based on the energetics of shallow living fishes cannot be used for grenadiers. -

Biology and Potential Use of Pacific Grenadier, Coryphaenoides Acrolepis, Off California
Biology and Potential Use of Pacific Grenadier, Coryphaenoides acrolepis, off California TETSUO MATSUI, SUSUMU KAT0 and SUSAN E. SMITH Introduction over65,OOOmetric tons(t)ofonespecies, grenadier, C. amtus;and giant grena- the roundnose grenadier, Coryphaenoi- dier, Albatrossiapectoralis. The Pacific Grenadiers (also knownas rattails) be- desrupestris, werecaught in 1975 (FAO, grenadier (Fig. 1) appears to have the long to the family Macrouridae, and are 1979). Although the catch of this species best potential, as the quality of its flesh is related tothecodfishes (family Gadidae). has declined substantially, other species good and it is abundant off California. They areamong themost abundant fishes are starting to be utilized, and the total The largest specimen of C. acrolepis we in continental slope and abyssal waters grenadier catch in 1986 was around have measured was over 95 cm (37 worldwide. The majority of macrourid 60,OOOt, 54percentofwhichwasround- inches) in total length. It weighed 4 kg species appear to spend a good part of the nose grenadier (FAO, 1988). Commer- (8.8 pounds) and was taken at lat. time swimming near the Ocean bottom, cial landings in the northeast Pacific have 29"31.3'N, long. 117" 12.0'W at a depth feeding on benthic and midwater organ- been minimal, even though macrourids of 1,050 fm (1,920 m). This may have isms (Marshall and Merrett, 1977). are the most abundant fishes found in been an unusually large individual, as the About 300 species are known, of which trawl catches in deep waters off Oregon prior known record length for the species 11 inhabit the deep waters off Califor- and Washington (Alton, 1972; Pearcy is smaller at 87 cm or 34 inches (Rass, nia'.