Deep-Sea Grenadiers and Climate Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

229 Index of Scientific and Vernacular Names
previous page 229 INDEX OF SCIENTIFIC AND VERNACULAR NAMES EXPLANATION OF THE SYSTEM Type faces used: Italics : Valid scientific names (genera and species) Italics : Synonyms * Italics : Misidentifications (preceded by an asterisk) ROMAN (saps) : Family names Roman : International (FAO) names of species 230 Page Page A African red snapper ................................................. 79 Abalistes stellatus ............................................... 42 African sawtail catshark ......................................... 144 Abámbolo ............................................................... 81 African sicklefìsh ...................................................... 62 Abámbolo de bajura ................................................ 81 African solenette .................................................... 111 Ablennes hians ..................................................... 44 African spadefish ..................................................... 63 Abuete cajeta ........................................................ 184 African spider shrimp ............................................. 175 Abuete de Angola ................................................. 184 African spoon-nose eel ............................................ 88 Abuete negro ........................................................ 184 African squid .......................................................... 199 Abuete real ........................................................... 183 African striped grunt ................................................ -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Otoliths in Situ in the Stem Teleost Cavenderichthys Talbragarensis
Journal of Vertebrate Paleontology ISSN: 0272-4634 (Print) 1937-2809 (Online) Journal homepage: https://www.tandfonline.com/loi/ujvp20 Otoliths in situ in the stem teleost Cavenderichthys talbragarensis (Woodward, 1895), otoliths in coprolites, and isolated otoliths from the Upper Jurassic of Talbragar, New South Wales, Australia Werner W. Schwarzhans, Timothy D. Murphy & Michael Frese To cite this article: Werner W. Schwarzhans, Timothy D. Murphy & Michael Frese (2018) Otoliths in situ in the stem teleost Cavenderichthystalbragarensis (Woodward, 1895), otoliths in coprolites, and isolated otoliths from the Upper Jurassic of Talbragar, New South Wales, Australia, Journal of Vertebrate Paleontology, 38:6, e1539740, DOI: 10.1080/02724634.2018.1539740 To link to this article: https://doi.org/10.1080/02724634.2018.1539740 © 2019 Werner W. Schwarzhans, Timothy View supplementary material D. Murphy, and Michael Frese. Published by Informa UK Limited, trading as Taylor & Francis Group. Published online: 19 Feb 2019. Submit your article to this journal Article views: 619 View related articles View Crossmark data Citing articles: 1 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ujvp20 Journal of Vertebrate Paleontology e1539740 (14 pages) © Published with license by the Society of Vertebrate Paleontology DOI: 10.1080/02724634.2018.1539740 ARTICLE OTOLITHS IN SITU IN THE STEM TELEOST CAVENDERICHTHYS TALBRAGARENSIS (WOODWARD, 1895), OTOLITHS IN COPROLITES, -

Macrouridae of the Eastern North Atlantic
FICHES D’IDENTIFICATION DU PLANCTON Edited by G.A. ROBINSON Institute for Marine Environmental Research Prospect Place, The Hoe, Plymouth PL1 3DH, England FICHE NO. 173/174/175 MACROURIDAE OF THE EASTERN NORTH ATLANTIC by N. R. MERRETT Institute of Oceanographic Sciences Natural Environment Research Council Wormley, Godalming, Surrey GU8 5UB, England (This publication may be referred to in the following form: Merrett, N. R. 1986. Macrouridae of the eastern North Atlantic. Fich. Ident. Plancton, 173/174/175,14 pp.) https://doi.org/10.17895/ices.pub.5155 INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA CONSEIL INTERNATIONAL POUR L’EXPLORATION DE LA MER Palzegade 2-4, DK-1261 Copenhagen K, Denmark 1986 ISSN 0109-2529 Figure 1. Egg and larval stages of Coelorinchus coelorinchus. (A) Egg from the Strait of Messina (1.20 mm diameter); (B) same on 4th day of incubation; (C) between 6th and 7th day; (D) at the end of 1 week; (E) newly hatched larva (4.21 mm TL); (F) larva 8 days old (3.88 mm TL); (G) larva 15 days old (464 mm TL) (redrawn from Sanzo, 1933); (H) 5.0 mm HL larva, “Thor” Stn 168 (51’30” ll”37’W). 2 . .. Figure 2. (A) Bathygadine, Gudornuc sp.: 30+ mm TL (redrawn from Fahay and Markle, 1984, Fig. 140B); Macrourur bcrglax: (B) 3-0 mm HL, “Dana” Stn 11995 (63’17“ 58’1 1’W); (C) 4.5 mm HL, “Dana” Stn 13044 (63’37“ 55’30’W); (D) 6-5 mm HL “Dana” Stn 13092 (58’45“ 42’14‘W); (E) 10.9 mm HL, “Dana” Stn 9985 (61’56” 37’30‘W). -

Response of Deep-Sea Scavengers to Ocean Acidification and the Odor from a Dead Grenadier
Vol. 350: 193–207, 2007 MARINE ECOLOGY PROGRESS SERIES Published November 22 doi: 10.3354/meps07188 Mar Ecol Prog Ser OPENPEN ACCESSCCESS Response of deep-sea scavengers to ocean acidification and the odor from a dead grenadier James P. Barry1,*, Jeffrey C. Drazen2 1Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 2University of Hawaii, Department of Oceanography, MSB 606, 1000 Pope Rd, Honolulu, Hawaii 96822, USA ABSTRACT: Experiments to assess the impact of ocean acidification on abyssal animals were per- formed off Central California. The survival of caged megafauna (Benthoctopus sp., Pachycara bulbi- ceps, Coryphaenoides armatus) exposed to CO2-rich and normal (control) seawater varied among species. Benthoctopus sp. and P. bulbiceps survived control conditions and month-long episodic exposure to acidic, CO2-rich waters (pH reductions of ~0.1 U). All C. armatus in both treatments died, potentially due to cage-related stress, predation, and exposure to acidic waters. High survival by P. bulbiceps and Benthoctopus under month-long exposure to CO2-rich waters indicates a physiologi- cal capacity to cope, at least temporarily, with stresses that will accompany expected future changes in ocean chemistry. The abundance of free-ranging scavengers was not correlated with variation in pH levels near fish cages. Incidental observations of abyssal scavengers collected using time-lapse cameras during these experiments were used secondarily to evaluate the hypothesis that macrourid fishes avoid the odor of dead conspecifics. Caged macrourids in view of time-lapse cameras died within 2 to 3 d, eliciting a strong response from the regional scavenger assemblage which aggregated near the cage. -

Betanodavirus and VER Disease: a 30-Year Research Review
pathogens Review Betanodavirus and VER Disease: A 30-year Research Review Isabel Bandín * and Sandra Souto Departamento de Microbioloxía e Parasitoloxía-Instituto de Acuicultura, Universidade de Santiago de Compostela, 15782 Santiago de Compostela, Spain; [email protected] * Correspondence: [email protected] Received: 20 December 2019; Accepted: 4 February 2020; Published: 9 February 2020 Abstract: The outbreaks of viral encephalopathy and retinopathy (VER), caused by nervous necrosis virus (NNV), represent one of the main infectious threats for marine aquaculture worldwide. Since the first description of the disease at the end of the 1980s, a considerable amount of research has gone into understanding the mechanisms involved in fish infection, developing reliable diagnostic methods, and control measures, and several comprehensive reviews have been published to date. This review focuses on host–virus interaction and epidemiological aspects, comprising viral distribution and transmission as well as the continuously increasing host range (177 susceptible marine species and epizootic outbreaks reported in 62 of them), with special emphasis on genotypes and the effect of global warming on NNV infection, but also including the latest findings in the NNV life cycle and virulence as well as diagnostic methods and VER disease control. Keywords: nervous necrosis virus (NNV); viral encephalopathy and retinopathy (VER); virus–host interaction; epizootiology; diagnostics; control 1. Introduction Nervous necrosis virus (NNV) is the causative agent of viral encephalopathy and retinopathy (VER), otherwise known as viral nervous necrosis (VNN). The disease was first described at the end of the 1980s in Australia and in the Caribbean [1–3], and has since caused a great deal of mortalities and serious economic losses in a variety of reared marine fish species, but also in freshwater species worldwide. -
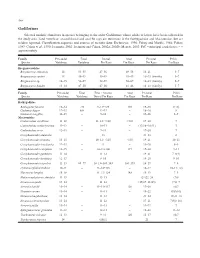
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

The Fish Fauna of Ampe`Re Seamount (NE Atlantic) and the Adjacent
Helgol Mar Res (2015) 69:13–23 DOI 10.1007/s10152-014-0413-4 ORIGINAL ARTICLE The fish fauna of Ampe`re Seamount (NE Atlantic) and the adjacent abyssal plain Bernd Christiansen • Rui P. Vieira • Sabine Christiansen • Anneke Denda • Frederico Oliveira • Jorge M. S. Gonc¸alves Received: 26 March 2014 / Revised: 15 September 2014 / Accepted: 24 September 2014 / Published online: 2 October 2014 Ó Springer-Verlag Berlin Heidelberg and AWI 2014 Abstract An inventory of benthic and benthopelagic stone’’ hypothesis of species dispersal, some differences fishes is presented as a result of two exploratory surveys can be attributed to the local features of the seamounts. around Ampe`re Seamount, between Madeira and the Por- tuguese mainland, covering water depths from 60 to Keywords Deep sea Á Fish distribution Á Ichthyofauna Á 4,400 m. A total of 239 fishes were collected using dif- Seamounts Á Zoogeography ferent types of sampling gear. Three chondrichthyan spe- cies and 31 teleosts in 21 families were identified. The collections showed a vertical zonation with little overlap, Introduction but indications for an affinity of species to certain water masses were only vague. Although most of the species Due to their vertical range and habitat diversity, seamounts present new records for Ampe`re Seamount, all of them often support high fish diversity, as compared to the sur- have been known for the NE Atlantic; endemic species rounding ocean, and some are known as hotspots of were not found. The comparison with fish communities at endemic species (e.g. Shank 2010; Stocks et al. 2012). other NE Atlantic seamounts indicates that despite a high Seamounts are considered to act as ‘‘stepping stones’’ for ichthyofaunal similarity, which supports the ‘‘stepping species dispersal (Almada et al. -

The Absence of Sharks from Abyssal Regions of the World's Oceans
Proc. R. Soc. B (2006) 273, 1435–1441 doi:10.1098/rspb.2005.3461 Published online 21 February 2006 The absence of sharks from abyssal regions of the world’s oceans Imants G. Priede1,*, Rainer Froese2, David M. Bailey3, Odd Aksel Bergstad4, Martin A. Collins5, Jan Erik Dyb6, Camila Henriques1, Emma G. Jones7 and Nicola King1 1University of Aberdeen, Oceanlab, Newburgh, Aberdeen AB41 6AA, UK 2Leibniz-Institut fu¨r Meereswissenschaften, IfM-GEOMAR, Du¨sternbrooker Weg 20, 24105 Kiel, Germany 3Marine Biology Research Division, Scripps Institution of Oceanography, UCSD 9500 Gilman Drive, La Jolla, CA 92093-0202, USA 4Institute of Marine Research, Flødevigen Marine Research Station, 4817 His, Norway 5British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 0ET, UK 6Møre Research, Section of Fisheries, PO Box 5075, 6021 Aalesund, Norway 7FRS Marine Laboratory, 375 Victoria Road, Aberdeen AB11 9DB, UK The oceanic abyss (depths greater than 3000 m), one of the largest environments on the planet, is characterized by absence of solar light, high pressures and remoteness from surface food supply necessitating special molecular, physiological, behavioural and ecological adaptations of organisms that live there. Sampling by trawl, baited hooks and cameras we show that the Chondrichthyes (sharks, rays and chimaeras) are absent from, or very rare in this region. Analysis of a global data set shows a trend of rapid disappearance of chondrichthyan species with depth when compared with bony fishes. Sharks, apparently well adapted to life at high pressures are conspicuous on slopes down to 2000 m including scavenging at food falls such as dead whales.