The Absence of Sharks from Abyssal Regions of the World's Oceans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morphology and Significance of the Luminous Organs in Alepocephaloid Fishes
Uiblein, F., Ott, J., Stachowitsch,©Akademie d. Wissenschaften M. (Eds), Wien; 1996: download Deep-sea unter www.biologiezentrum.at and extreme shallow-water habitats: affinities and adaptations. - Biosystematics and Ecology Series 11:151-163. Morphology and significance of the luminous organs in alepocephaloid fishes Y. I. SAZONOV Abstract: Alepocephaloid fishes, or slickheads (two families, Alepocephalidae and Platytroctidae), are deep-sea fishes distributed in all three major oceans at depths of ca. 100-5000 m, usually between ca. 500 and 3000 m. Among about 150 known species, 13 alepocephalid and all (ca. 40) platytroctid species have diverse light organs: 1) postcleithral luminous gland (all platytroctids); it releases a luminescent secredon which presumably startles or blinds predators and allows the fish to escape; 2) relatively large, regulär photophores on the head and ventral parts of the body in the alepocephalid Microphotolepis and in 6 (of 14) platytroctid genera. These organs may serve for countershading and possibly for giving signals to other individuals of the same species; 3) small "simple" or "secondary" photophores covering the whole body and fins in 5 alepocephalid genera, and a few such structures in 1 platytroctid; these organs may be used for Camouflage in the glow of spontaneous bioluminescence; 4) the mental light organ in 2 alepocephalid genera from the abyssal zone (Bathyprion and Mirognathus) may be used as a Iure to attract prey. Introduction Alepocephaloid fishes, or slickheads, comprise two families of isospondyl- ous fishes (Platytroctidae and Alepocephalidae). The group is one of the most diverse among oceanic bathypelagic fishes (about 35 genera with 150 species), and these fishes play a significant role in the communities of meso- and bathypelagic animals. -

Cusk Eels, Brotulas [=Cherublemma Trotter [E
FAMILY Ophidiidae Rafinesque, 1810 - cusk eels SUBFAMILY Ophidiinae Rafinesque, 1810 - cusk eels [=Ofidini, Otophidioidei, Lepophidiinae, Genypterinae] Notes: Ofidini Rafinesque, 1810b:38 [ref. 3595] (ordine) Ophidion [as Ophidium; latinized to Ophididae by Bonaparte 1831:162, 184 [ref. 4978] (family); stem corrected to Ophidi- by Lowe 1843:92 [ref. 2832], confirmed by Günther 1862a:317, 370 [ref. 1969], by Gill 1872:3 [ref. 26254] and by Carus 1893:578 [ref. 17975]; considered valid with this authorship by Gill 1893b:136 [ref. 26255], by Goode & Bean 1896:345 [ref. 1848], by Nolf 1985:64 [ref. 32698], by Patterson 1993:636 [ref. 32940] and by Sheiko 2013:63 [ref. 32944] Article 11.7.2; family name sometimes seen as Ophidionidae] Otophidioidei Garman, 1899:390 [ref. 1540] (no family-group name) Lepophidiinae Robins, 1961:218 [ref. 3785] (subfamily) Lepophidium Genypterinae Lea, 1980 (subfamily) Genypterus [in unpublished dissertation: Systematics and zoogeography of cusk-eels of the family Ophidiidae, subfamily Ophidiinae, from the eastern Pacific Ocean, University of Miami, not available] GENUS Cherublemma Trotter, 1926 - cusk eels, brotulas [=Cherublemma Trotter [E. S.], 1926:119, Brotuloides Robins [C. R.], 1961:214] Notes: [ref. 4466]. Neut. Cherublemma lelepris Trotter, 1926. Type by monotypy. •Valid as Cherublemma Trotter, 1926 -- (Pequeño 1989:48 [ref. 14125], Robins in Nielsen et al. 1999:27, 28 [ref. 24448], Castellanos-Galindo et al. 2006:205 [ref. 28944]). Current status: Valid as Cherublemma Trotter, 1926. Ophidiidae: Ophidiinae. (Brotuloides) [ref. 3785]. Masc. Leptophidium emmelas Gilbert, 1890. Type by original designation (also monotypic). •Synonym of Cherublemma Trotter, 1926 -- (Castro-Aguirre et al. 1993:80 [ref. 21807] based on placement of type species, Robins in Nielsen et al. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Fishes Scales & Tails Scale Types 1
Phylum Chordata SUBPHYLUM VERTEBRATA Metameric chordates Linear series of cartilaginous or boney support (vertebrae) surrounding or replacing the notochord Expanded anterior portion of nervous system THE FISHES SCALES & TAILS SCALE TYPES 1. COSMOID (most primitive) First found on ostracaderm agnathans, thick & boney - composed of: Ganoine (enamel outer layer) Cosmine (thick under layer) Spongy bone Lamellar bone Perhaps selected for protection against eurypterids, but decreased flexibility 2. GANOID (primitive, still found on some living fish like gar) 3. PLACOID (old scale type found on the chondrichthyes) Dentine, tooth-like 4. CYCLOID (more recent scale type, found in modern osteichthyes) 5. CTENOID (most modern scale type, found in modern osteichthyes) TAILS HETEROCERCAL (primitive, still found on chondrichthyes) ABBREVIATED HETEROCERCAL (found on some primitive living fish like gar) DIPHYCERCAL (primitive, found on sarcopterygii) HOMOCERCAL (most modern, found on most modern osteichthyes) Agnatha (class) [connect the taxa] Cyclostomata (order) Placodermi Acanthodii (class) (class) Chondrichthyes (class) Osteichthyes (class) Actinopterygii (subclass) Sarcopterygii (subclass) Dipnoi (order) Crossopterygii (order) Ripidistia (suborder) Coelacanthiformes (suborder) Chondrostei (infra class) Holostei (infra class) Teleostei (infra class) CLASS AGNATHA ("without jaws") Most primitive - first fossils in Ordovician Bottom feeders, dorsal/ventral flattened Cosmoid scales (Ostracoderms) Pair of eyes + pineal eye - present in a few living fish and reptiles - regulates circadian rhythms Nine - seven gill pouches No paired appendages, medial nosril ORDER CYCLOSTOMATA (60 spp) Last living representatives - lampreys & hagfish Notochord not replaced by vertebrae Cartilaginous cranium, scaleless body Sea lamprey predaceous - horny teeth in buccal cavity & on tongue - secretes anti-coaggulant Lateral Line System No stomach or spleen 5 - 7 year life span - adults move into freshwater streams, spawn, & die. -

Zootaxa, Marine Fish Diversity: History of Knowledge and Discovery
Zootaxa 2525: 19–50 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) Marine fish diversity: history of knowledge and discovery (Pisces) WILLIAM N. ESCHMEYER1, 5, RONALD FRICKE2, JON D. FONG3 & DENNIS A. POLACK4 1Curator emeritus, California Academy of Sciences, San Francisco, California, U.S.A. 94118 and Research Associate, Florida Museum of Natural History, Gainesville, Florida, U.S.A. 32611. E-mail: [email protected] 2Ichthyology, Staatliches Museum für Naturkunde, Rosenstein 1, 70191 Stuttgart, Germany. E-mail: [email protected] 3California Academy of Sciences, San Francisco, California, U.S.A. 94118. E-mail: [email protected] 4P.O. Box 518, Halfway House 1685, South Africa. E-mail: [email protected] 5Corresponding Author. E-mail: [email protected] Abstract The increase in knowledge of marine fish biodiversity over the last 250 years is assessed. The Catalog of Fishes database (http://research.calacademy.org/ichthyology/catalog) on which this study is based, has been maintained for 25 years and includes information on more than 50,000 available species names of fishes, with more than 31,000 of them currently regarded as valid species. New marine species are being described at a rate of about 100–150 per year, with freshwater numbers slightly higher. In addition, over 10,000 generic names are available ones of which 3,118 are deemed valid for marine fishes (as of Feb. 19, 2010). This report concentrates on fishes with at least some stage of their life cycle in the sea. The number of valid marine species, about 16,764 (Feb. -

Otoliths in Situ in the Stem Teleost Cavenderichthys Talbragarensis
Journal of Vertebrate Paleontology ISSN: 0272-4634 (Print) 1937-2809 (Online) Journal homepage: https://www.tandfonline.com/loi/ujvp20 Otoliths in situ in the stem teleost Cavenderichthys talbragarensis (Woodward, 1895), otoliths in coprolites, and isolated otoliths from the Upper Jurassic of Talbragar, New South Wales, Australia Werner W. Schwarzhans, Timothy D. Murphy & Michael Frese To cite this article: Werner W. Schwarzhans, Timothy D. Murphy & Michael Frese (2018) Otoliths in situ in the stem teleost Cavenderichthystalbragarensis (Woodward, 1895), otoliths in coprolites, and isolated otoliths from the Upper Jurassic of Talbragar, New South Wales, Australia, Journal of Vertebrate Paleontology, 38:6, e1539740, DOI: 10.1080/02724634.2018.1539740 To link to this article: https://doi.org/10.1080/02724634.2018.1539740 © 2019 Werner W. Schwarzhans, Timothy View supplementary material D. Murphy, and Michael Frese. Published by Informa UK Limited, trading as Taylor & Francis Group. Published online: 19 Feb 2019. Submit your article to this journal Article views: 619 View related articles View Crossmark data Citing articles: 1 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ujvp20 Journal of Vertebrate Paleontology e1539740 (14 pages) © Published with license by the Society of Vertebrate Paleontology DOI: 10.1080/02724634.2018.1539740 ARTICLE OTOLITHS IN SITU IN THE STEM TELEOST CAVENDERICHTHYS TALBRAGARENSIS (WOODWARD, 1895), OTOLITHS IN COPROLITES, -

Class Wars: Chondrichthyes and Osteichthyes Dominance in Chesapeake Bay, 2002-2012
Class Wars: Chondrichthyes and Osteichthyes dominance in Chesapeake Bay, 2002-2012. 01 July 2013 Introduction The objective of this analysis was to demonstrate a possible changing relationship between two Classes of fishes, Osteichthyes (the bony fishes) and Chondrichthyes (the cartilaginous fishes) in Chesapeake Bay based on 11 years of monitoring. If any changes between the two Classes appeared to be significant, either statistically or anecdotally, the data were explored further in an attempt to explain the variation. The Class Osteichthyes is characterized by having a skeleton made of bone and is comprised of the majority of fish species worldwide, while the Chondrichthyes skeleton is made of cartilage and is represented by the sharks, skates, and rays (the elasmobranch fishes) and chimaeras1. Many shark species are generally categorized as apex predators, while skates and rays and some smaller sharks can be placed into the mesopredator functional group (Myers et al., 2007). By definition, mesopredators prey upon a significant array of lower trophic groups, but also serve as the prey base for apex predators. Global demand for shark and consequential shark fishing mortality, estimated at 97 million sharks in 2010 (Worm et al., 2013), is hypothesized to have contributed to the decline of these apex predators in recent years (Baum et al., 2003 and Fowler et al., 2005), which in turn is suggested to have had a cascading effect on lower trophic levels—an increase in mesopredators and subsequent decrease in the prey base (Myers et al., 2007). According to 10 years of trawl survey monitoring of Chesapeake Bay, fish species composition of catches has shown a marked change over the years (Buchheister et al., 2013). -

Response of Deep-Sea Scavengers to Ocean Acidification and the Odor from a Dead Grenadier
Vol. 350: 193–207, 2007 MARINE ECOLOGY PROGRESS SERIES Published November 22 doi: 10.3354/meps07188 Mar Ecol Prog Ser OPENPEN ACCESSCCESS Response of deep-sea scavengers to ocean acidification and the odor from a dead grenadier James P. Barry1,*, Jeffrey C. Drazen2 1Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 2University of Hawaii, Department of Oceanography, MSB 606, 1000 Pope Rd, Honolulu, Hawaii 96822, USA ABSTRACT: Experiments to assess the impact of ocean acidification on abyssal animals were per- formed off Central California. The survival of caged megafauna (Benthoctopus sp., Pachycara bulbi- ceps, Coryphaenoides armatus) exposed to CO2-rich and normal (control) seawater varied among species. Benthoctopus sp. and P. bulbiceps survived control conditions and month-long episodic exposure to acidic, CO2-rich waters (pH reductions of ~0.1 U). All C. armatus in both treatments died, potentially due to cage-related stress, predation, and exposure to acidic waters. High survival by P. bulbiceps and Benthoctopus under month-long exposure to CO2-rich waters indicates a physiologi- cal capacity to cope, at least temporarily, with stresses that will accompany expected future changes in ocean chemistry. The abundance of free-ranging scavengers was not correlated with variation in pH levels near fish cages. Incidental observations of abyssal scavengers collected using time-lapse cameras during these experiments were used secondarily to evaluate the hypothesis that macrourid fishes avoid the odor of dead conspecifics. Caged macrourids in view of time-lapse cameras died within 2 to 3 d, eliciting a strong response from the regional scavenger assemblage which aggregated near the cage. -

Systematic Morphology of Fishes in the Early 21St Century
Copeia 103, No. 4, 2015, 858–873 When Tradition Meets Technology: Systematic Morphology of Fishes in the Early 21st Century Eric J. Hilton1, Nalani K. Schnell2, and Peter Konstantinidis1 Many of the primary groups of fishes currently recognized have been established through an iterative process of anatomical study and comparison of fishes that has spanned a time period approaching 500 years. In this paper we give a brief history of the systematic morphology of fishes, focusing on some of the individuals and their works from which we derive our own inspiration. We further discuss what is possible at this point in history in the anatomical study of fishes and speculate on the future of morphology used in the systematics of fishes. Beyond the collection of facts about the anatomy of fishes, morphology remains extremely relevant in the age of molecular data for at least three broad reasons: 1) new techniques for the preparation of specimens allow new data sources to be broadly compared; 2) past morphological analyses, as well as new ideas about interrelationships of fishes (based on both morphological and molecular data) provide rich sources of hypotheses to test with new morphological investigations; and 3) the use of morphological data is not limited to understanding phylogeny and evolution of fishes, but rather is of broad utility to understanding the general biology (including phenotypic adaptation, evolution, ecology, and conservation biology) of fishes. Although in some ways morphology struggles to compete with the lure of molecular data for systematic research, we see the anatomical study of fishes entering into a new and exciting phase of its history because of recent technological and methodological innovations. -

Deep-Sea Life Issue 14, January 2020 Cruise News E/V Nautilus Telepresence Exploration of the U.S
Deep-Sea Life Issue 14, January 2020 Welcome to the 14th edition of Deep-Sea Life (a little later than anticipated… such is life). As always there is bound to be something in here for everyone. Illustrated by stunning photography throughout, learn about the deep-water canyons of Lebanon, remote Pacific Island seamounts, deep coral habitats of the Caribbean Sea, Gulf of Mexico, Southeast USA and the North Atlantic (with good, bad and ugly news), first trials of BioCam 3D imaging technology (very clever stuff), new deep pelagic and benthic discoveries from the Bahamas, high-risk explorations under ice in the Arctic (with a spot of astrobiology thrown in), deep-sea fauna sensitivity assessments happening in the UK and a new photo ID guide for mesopelagic fish. Read about new projects to study unexplored areas of the Mid-Atlantic Ridge and Azores Plateau, plans to develop a water-column exploration programme, and assessment of effects of ice shelf collapse on faunal assemblages in the Antarctic. You may also be interested in ongoing projects to address and respond to governance issues and marine conservation. It’s all here folks! There are also reports from past meetings and workshops related to deep seabed mining, deep-water corals, deep-water sharks and rays and information about upcoming events in 2020. Glance over the many interesting new papers for 2019 you may have missed, the scientist profiles, job and publishing opportunities and the wanted section – please help your colleagues if you can. There are brief updates from the Deep- Ocean Stewardship Initiative and for the deep-sea ecologists amongst you, do browse the Deep-Sea Biology Society president’s letter. -
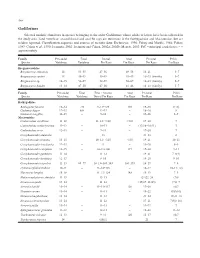
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

The Fish Fauna of Ampe`Re Seamount (NE Atlantic) and the Adjacent
Helgol Mar Res (2015) 69:13–23 DOI 10.1007/s10152-014-0413-4 ORIGINAL ARTICLE The fish fauna of Ampe`re Seamount (NE Atlantic) and the adjacent abyssal plain Bernd Christiansen • Rui P. Vieira • Sabine Christiansen • Anneke Denda • Frederico Oliveira • Jorge M. S. Gonc¸alves Received: 26 March 2014 / Revised: 15 September 2014 / Accepted: 24 September 2014 / Published online: 2 October 2014 Ó Springer-Verlag Berlin Heidelberg and AWI 2014 Abstract An inventory of benthic and benthopelagic stone’’ hypothesis of species dispersal, some differences fishes is presented as a result of two exploratory surveys can be attributed to the local features of the seamounts. around Ampe`re Seamount, between Madeira and the Por- tuguese mainland, covering water depths from 60 to Keywords Deep sea Á Fish distribution Á Ichthyofauna Á 4,400 m. A total of 239 fishes were collected using dif- Seamounts Á Zoogeography ferent types of sampling gear. Three chondrichthyan spe- cies and 31 teleosts in 21 families were identified. The collections showed a vertical zonation with little overlap, Introduction but indications for an affinity of species to certain water masses were only vague. Although most of the species Due to their vertical range and habitat diversity, seamounts present new records for Ampe`re Seamount, all of them often support high fish diversity, as compared to the sur- have been known for the NE Atlantic; endemic species rounding ocean, and some are known as hotspots of were not found. The comparison with fish communities at endemic species (e.g. Shank 2010; Stocks et al. 2012). other NE Atlantic seamounts indicates that despite a high Seamounts are considered to act as ‘‘stepping stones’’ for ichthyofaunal similarity, which supports the ‘‘stepping species dispersal (Almada et al.