Gadiformes: Macrouridae) of the Genus Coryphaenoides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Faunal Assemblage Structure on the Patton Seamount (Gulf of Alaska, USA)
Faunal Assemblage Structure on the Patton Seamount (Gulf of Alaska, USA) Gerald R. Hoff and Bradley Stevens Reprinted from the Alaska Fishery Research Bulletin Vol. 11 No. 1, Summer 2005 The Alaska Fisheries Research Bulletin can be found on the World Wide Web at URL: http://www.adfg.state.ak.us/pubs/afrb/afrbhome.php Alaska Fishery Research Bulletin 11(1):27–36. 2005. Copyright © 2005 by the Alaska Department of Fish and Game Faunal Assemblage Structure on the Patton Seamount (Gulf of Alaska, USA) Gerald R. Hoff and Bradley Stevens ABSTRACT: Epibenthic and demersal assemblages of fish and invertebrates on the Patton Seamount in the Gulf of Alaska, U.S.A., were studied in July 1999 using the Deep Sea Research Vehicle Alvin. Faunal associations with depth were described using video analysis of 8 dives from 151 to 3,375 m. A cluster analysis applied to the observations suggests three benthic faunal communities based on depth: 1) a shallow-water community (151–950 m) consisting mainly of rockfishes, flatfishes, sea stars, and attached suspension feeders, 2) a mid-depth community (400–1500 m) also consisting of numerous attached suspension-feeding organisms such as corals, sponges, crinoids, sea anemones, and sea cucumbers and fish such as the sablefishAnoplopoma fimbria and the giant grenadier Albatrossia pectoralis both of which were aggregated over a relatively narrow depth range, and 3) a deep-water community (500–3,375 m) consisting of fewer attached suspension feeders and more highly mobile species such as the Pacific grenadier Coryphaenoides acrolepis, popeye grenadier C. cinereus, Pacific flatnose Antimora microlepis, and large mobile crabs Macroregonia macrochira and Chionoecetes spp. -

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

RADIOMETRIC AGE DETERMINATION of the GIANT GRENADIER (A/Batrossia Pectoralis) USING 210Pb: 226Ra DISEQUILIBRIA
I . RADIOMETRIC AGE DETERMINATION OF THE GIANT GRENADIER (A/batrossia pectoralis) USING 210Pb: 226Ra DISEQUILIBRIA A thesis submitted to the faculty of San Francisco State University in partial fulfillment of the requirements for the degree Master of Science in Marine Science by Erica Janis Burton San Francisco, California December, 1999 Copyright by Erica Janis Burton 1999 RADIOMETRIC AGE DETERMINATION OF THE GIANT GRENADIER (Aibatrossia pectoralis) USING 210Pb: 226 Ra DISEQUILIBRIA Erica Janis Burton San Francisco State University 1999 Age estimates determined from growth increments in sagittal otolith sections indicated that Albatrossia pectoralis is slow growing (K :<:: 0.023) and lives up to 56 years. Growth increments found in otolith sections, however, were difficult to interpret. The von Bertalanffy growth function for A. pectoralis otolith section age estimates did not fit size-at-age data well. To validate age and longevity estimates, ages were determined using the radioactive disequilibria of 210Pb: 226 Ra in otolith cores from adult A. pectoralis. Radiometric and growth increment ages agreed for 6 of the 12 pooled otolith age-groups. Radiometric age determination confirmed longevity to at least 32 years for females and 27 years for males. Additional age and longevity estimates are still necessary to develop an informed fishery management plan for A. pectoralis. I certify that the Abstract is a correct representation of the content of this thesis. ~~~-~ (Date) - ACKNOWLEDGEMENTS Although a thesis is authored by one person, it is not accomplished alone. I have many people to thank who contributed their time, sweat, and talent. I sincerely thank my thesis committee members. Dr. -

Otoliths in Situ in the Stem Teleost Cavenderichthys Talbragarensis
Journal of Vertebrate Paleontology ISSN: 0272-4634 (Print) 1937-2809 (Online) Journal homepage: https://www.tandfonline.com/loi/ujvp20 Otoliths in situ in the stem teleost Cavenderichthys talbragarensis (Woodward, 1895), otoliths in coprolites, and isolated otoliths from the Upper Jurassic of Talbragar, New South Wales, Australia Werner W. Schwarzhans, Timothy D. Murphy & Michael Frese To cite this article: Werner W. Schwarzhans, Timothy D. Murphy & Michael Frese (2018) Otoliths in situ in the stem teleost Cavenderichthystalbragarensis (Woodward, 1895), otoliths in coprolites, and isolated otoliths from the Upper Jurassic of Talbragar, New South Wales, Australia, Journal of Vertebrate Paleontology, 38:6, e1539740, DOI: 10.1080/02724634.2018.1539740 To link to this article: https://doi.org/10.1080/02724634.2018.1539740 © 2019 Werner W. Schwarzhans, Timothy View supplementary material D. Murphy, and Michael Frese. Published by Informa UK Limited, trading as Taylor & Francis Group. Published online: 19 Feb 2019. Submit your article to this journal Article views: 619 View related articles View Crossmark data Citing articles: 1 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ujvp20 Journal of Vertebrate Paleontology e1539740 (14 pages) © Published with license by the Society of Vertebrate Paleontology DOI: 10.1080/02724634.2018.1539740 ARTICLE OTOLITHS IN SITU IN THE STEM TELEOST CAVENDERICHTHYS TALBRAGARENSIS (WOODWARD, 1895), OTOLITHS IN COPROLITES, -
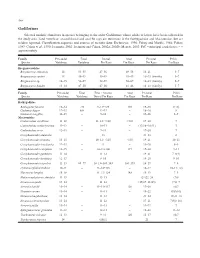
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

The Absence of Sharks from Abyssal Regions of the World's Oceans
Proc. R. Soc. B (2006) 273, 1435–1441 doi:10.1098/rspb.2005.3461 Published online 21 February 2006 The absence of sharks from abyssal regions of the world’s oceans Imants G. Priede1,*, Rainer Froese2, David M. Bailey3, Odd Aksel Bergstad4, Martin A. Collins5, Jan Erik Dyb6, Camila Henriques1, Emma G. Jones7 and Nicola King1 1University of Aberdeen, Oceanlab, Newburgh, Aberdeen AB41 6AA, UK 2Leibniz-Institut fu¨r Meereswissenschaften, IfM-GEOMAR, Du¨sternbrooker Weg 20, 24105 Kiel, Germany 3Marine Biology Research Division, Scripps Institution of Oceanography, UCSD 9500 Gilman Drive, La Jolla, CA 92093-0202, USA 4Institute of Marine Research, Flødevigen Marine Research Station, 4817 His, Norway 5British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 0ET, UK 6Møre Research, Section of Fisheries, PO Box 5075, 6021 Aalesund, Norway 7FRS Marine Laboratory, 375 Victoria Road, Aberdeen AB11 9DB, UK The oceanic abyss (depths greater than 3000 m), one of the largest environments on the planet, is characterized by absence of solar light, high pressures and remoteness from surface food supply necessitating special molecular, physiological, behavioural and ecological adaptations of organisms that live there. Sampling by trawl, baited hooks and cameras we show that the Chondrichthyes (sharks, rays and chimaeras) are absent from, or very rare in this region. Analysis of a global data set shows a trend of rapid disappearance of chondrichthyan species with depth when compared with bony fishes. Sharks, apparently well adapted to life at high pressures are conspicuous on slopes down to 2000 m including scavenging at food falls such as dead whales. -

Comparison of Size Selectivity Between Marine Mammals and Commercial Fisheries with Recommendations for Restructuring Management Policies
NOAA Technical Memorandum NMFS-AFSC-159 Comparison of Size Selectivity Between Marine Mammals and Commercial Fisheries with Recommendations for Restructuring Management Policies by M. A. Etnier and C. W. Fowler U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Alaska Fisheries Science Center October 2005 NOAA Technical Memorandum NMFS The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series reflect sound professional work and may be referenced in the formal scientific and technical literature. The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-NWFSC series is currently used by the Northwest Fisheries Science Center. This document should be cited as follows: Etnier, M. A., and C. W. Fowler. 2005. Comparison of size selectivity between marine mammals and commercial fisheries with recommendations for restructuring management policies. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-159, 274 p. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. NOAA Technical Memorandum NMFS-AFSC-159 Comparison of Size Selectivity Between Marine Mammals and Commercial Fisheries with Recommendations for Restructuring Management Policies by M. A. Etnier and C. W. Fowler Alaska Fisheries Science Center 7600 Sand Point Way N.E. Seattle, WA 98115 www.afsc.noaa.gov U.S. DEPARTMENT OF COMMERCE Carlos M. -

RACE Species Codes and Survey Codes 2018
Alaska Fisheries Science Center Resource Assessment and Conservation Engineering MAY 2019 GROUNDFISH SURVEY & SPECIES CODES U.S. Department of Commerce | National Oceanic and Atmospheric Administration | National Marine Fisheries Service SPECIES CODES Resource Assessment and Conservation Engineering Division LIST SPECIES CODE PAGE The Species Code listings given in this manual are the most complete and correct 1 NUMERICAL LISTING 1 copies of the RACE Division’s central Species Code database, as of: May 2019. This OF ALL SPECIES manual replaces all previous Species Code book versions. 2 ALPHABETICAL LISTING 35 OF FISHES The source of these listings is a single Species Code table maintained at the AFSC, Seattle. This source table, started during the 1950’s, now includes approximately 2651 3 ALPHABETICAL LISTING 47 OF INVERTEBRATES marine taxa from Pacific Northwest and Alaskan waters. SPECIES CODE LIMITS OF 4 70 in RACE division surveys. It is not a comprehensive list of all taxa potentially available MAJOR TAXONOMIC The Species Code book is a listing of codes used for fishes and invertebrates identified GROUPS to the surveys nor a hierarchical taxonomic key. It is a linear listing of codes applied GROUNDFISH SURVEY 76 levelsto individual listed under catch otherrecords. codes. Specifically, An individual a code specimen assigned is to only a genus represented or higher once refers by CODES (Appendix) anyto animals one code. identified only to that level. It does not include animals identified to lower The Code listing is periodically reviewed -

Biology and Potential Use of Pacific Grenadier, Coryphaenoides Acrolepis, Off California
Biology and Potential Use of Pacific Grenadier, Coryphaenoides acrolepis, off California TETSUO MATSUI, SUSUMU KAT0 and SUSAN E. SMITH Introduction over65,OOOmetric tons(t)ofonespecies, grenadier, C. amtus;and giant grena- the roundnose grenadier, Coryphaenoi- dier, Albatrossiapectoralis. The Pacific Grenadiers (also knownas rattails) be- desrupestris, werecaught in 1975 (FAO, grenadier (Fig. 1) appears to have the long to the family Macrouridae, and are 1979). Although the catch of this species best potential, as the quality of its flesh is related tothecodfishes (family Gadidae). has declined substantially, other species good and it is abundant off California. They areamong themost abundant fishes are starting to be utilized, and the total The largest specimen of C. acrolepis we in continental slope and abyssal waters grenadier catch in 1986 was around have measured was over 95 cm (37 worldwide. The majority of macrourid 60,OOOt, 54percentofwhichwasround- inches) in total length. It weighed 4 kg species appear to spend a good part of the nose grenadier (FAO, 1988). Commer- (8.8 pounds) and was taken at lat. time swimming near the Ocean bottom, cial landings in the northeast Pacific have 29"31.3'N, long. 117" 12.0'W at a depth feeding on benthic and midwater organ- been minimal, even though macrourids of 1,050 fm (1,920 m). This may have isms (Marshall and Merrett, 1977). are the most abundant fishes found in been an unusually large individual, as the About 300 species are known, of which trawl catches in deep waters off Oregon prior known record length for the species 11 inhabit the deep waters off Califor- and Washington (Alton, 1972; Pearcy is smaller at 87 cm or 34 inches (Rass, nia'. -

Seafood Watch Seafood Report
Seafood Watch Seafood Report Grenadier Giant grenadier (Albatrossia pectoralis) Pacific grenadier (Coryphaenoides acrolepis) Shoulderspot grenadier (Coelorinchus scaphopsis) Popeye grenadier (Coryphaenoides cinereus) Smooth grenadier (Nezumia liolepis) California grenadier (Nezumia stelgidolepis) (Pacific Grenadier, courtesy of ODFW) West Coast Region Final Report November 23, 2005 Updated March 17, 2006 Kelsey Abbott Independent Contractor MBA_SeafoodWatch_GrenadierReport_FinalReport.doc March 17, 2006 About Seafood Watch® and the Seafood Reports Monterey Bay Aquarium’s Seafood Watch® program evaluates the ecological sustainability of wild-caught and farmed seafood commonly found in the United States marketplace. Seafood Watch® defines sustainable seafood as originating from sources, whether wild-caught or farmed, which can maintain or increase production in the long-term without jeopardizing the structure or function of affected ecosystems. Seafood Watch® makes its science-based recommendations available to the public in the form of regional pocket guides that can be downloaded from the Internet (seafoodwatch.org) or obtained from the Seafood Watch® program by emailing [email protected]. The program’s goals are to raise awareness of important ocean conservation issues and empower seafood consumers and businesses to make choices for healthy oceans. Each sustainability recommendation on the regional pocket guides is supported by a Seafood Report. Each report synthesizes and analyzes the most current ecological, fisheries and ecosystem science on a species, then evaluates this information against the program’s conservation ethic to arrive at a recommendation of “Best Choices,” “Good Alternatives,” or “Avoid.” The detailed evaluation methodology is available upon request. In producing the Seafood Reports, Seafood Watch® seeks out research published in academic, peer-reviewed journals whenever possible. -

2015 Gulf of Alaska Bottom Trawl Survey
NOAA Technical Memorandum NMFS-AFSC-325 doi:10.7289/V5/TM-AFSC-325 Data Report: 2015 Gulf of Alaska Bottom Trawl Survey P. G. von Szalay and N. W. Raring U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Alaska Fisheries Science Center August 2016 NOAA Technical Memorandum NMFS The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series reflect sound professional work and may be referenced in the formal scientific and technical literature. The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-NWFSC series is currently used by the Northwest Fisheries Science Center. This document should be cited as follows: von Szalay, P. G., and N. W. Raring. 2016. Data report: 2015 Gulf of Alaska bottom trawl survey. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-325, 249 p. Document available: http://www.afsc.noaa.gov/Publications/AFSC-TM/NOAA-TM-AFSC-325.pdf Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. NOAA Technical Memorandum NMFS-AFSC-325 doi:10.7289/V5/TM-AFSC-325 Data Report: 2015 Gulf of Alaska Bottom Trawl Survey P. G. von Szalay and N. W. Raring Alaska Fisheries Science Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 7600 Sand Point Way NE Seattle, WA 98115 www.afsc.noaa.gov U.S.