Modeling SARS-Cov-2/COVID-19 Growth in Germany at the County Level
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Konfirmationen 2021
Evangelische Kirchengemeinde Gangelt, Selfkant, Waldfeucht Gemeindebrief September/ Oktober/ November 2021 Konfirmationen 2021 1 Dürfen wir vorstellen: Konfirmation ist am Sonntag, 03.10.2021 im Geusen-Haus zu Bocket, An der Flachsroth 4 in 52525 Waldfeucht-Bocket Die Amtshandlungen werden nur in der Print-Ausgabe veröffentlicht 2 Foto: B. Hoppe Ein Wort zuvor Liebe Leserin, Foto: privat lieber Leser! Liebe Gemeinde, mit diesen Zeilen erreicht Sie die Herbstausgabe unseres Gemeindebriefs. Nach wie vor ist es eine verkürzte Ausgabe. Und doch enthält er das ganze Spektrum menschlichen Lebens. Wir sind angefüllt von den Katastrophenbildern vergangener Wochen. Auch Menschen und Gemeinden unseres Kirchenkreises wurde von den Hochwassern schwer getroffen. Die Corona-Lage bleibt unübersichtlich. Wenn auch Gottesdienste stattfinden können und auch die Unterrichte hoffentlich wieder präsentisch stattfinden werden, so ist die Zahl der Gemeindeveranstaltungen recht übersichtlich. Das liegt auch an unserem Bauvorhaben in Gangelt. Das Gemeindezentrum Friedenskirche ist nun erst einmal still gelegt. Allein das Gemeindebüro bleibt für Sie geöffnet. Die Redaktion hat sich entschieden, mit dem Stichwort „Konfirmationen 2021“ eine schöne Seite unseres Gemeindelebens in den Vordergrund zu rücken. Wir werden am 3. Oktober 14 junge Menschen in die Freiheit und Verantwortung des Glaubens entsenden und dieses in den Gottesdiensten feiern. Darauf dürfen wir uns gemeinsam freuen. Ihnen und Ihren Lieben Gesundheit, Lebensfreude und Gottes reichen Segen, Ihr Pfarrer Mathias Schoenen 3 Inhalt Unsere Konfirmanden/innen 2 Ein Wort zuvor 3 Andacht 5 Jubiläum Weltladen 6 Wir bauen ein Haus 7 Abschied v. K.-H. Palmen 8 Gemeindegruppen 9 Aus der Region 10 Brief des Präses 11 Aus dem Kirchenkreis 12 Adressen 14 Amtshandlungen 15 Gottesdienste 16 Impressum Gemeindebrief der Evangelischen Kirchengemeinde Gangelt, Selfkant, Waldfeucht Herausgeber: Presbyterium Redaktion: Lothar Kötz, Dr. -

The Germans of Roberts Cove, Louisiana: German Rice Cultivation and the Making of a German-American Community in Acadia Parish, 1881-1917
University of New Orleans ScholarWorks@UNO University of New Orleans Theses and Dissertations Dissertations and Theses 12-17-2010 The Germans of Roberts Cove, Louisiana: German Rice Cultivation and the Making of a German-American Community in Acadia Parish, 1881-1917 Lydia Soileau University of New Orleans Follow this and additional works at: https://scholarworks.uno.edu/td Recommended Citation Soileau, Lydia, "The Germans of Roberts Cove, Louisiana: German Rice Cultivation and the Making of a German-American Community in Acadia Parish, 1881-1917" (2010). University of New Orleans Theses and Dissertations. 1246. https://scholarworks.uno.edu/td/1246 This Thesis is protected by copyright and/or related rights. It has been brought to you by ScholarWorks@UNO with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights- holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Thesis has been accepted for inclusion in University of New Orleans Theses and Dissertations by an authorized administrator of ScholarWorks@UNO. For more information, please contact [email protected]. The Germans of Roberts Cove, Louisiana: German Rice Cultivation and the Making of a German-American Community in Acadia Parish, 1881-1917 A Thesis Submitted to the Graduate Faculty of the University of New Orleans in partial fulfillment of the requirements for the degree of Master of Arts In History By Lydia Alicia Soileau B.A. -

2 Years 255 Days
Volume 29, No. 9 NATO Air Base Geilenkirchen 27 Septembery 2013 Photo by Wiel Borghans Exercise, Exercise, Exercise The E-3A Component held a large, multi-scenario exercise in cooridination with local German emergency personnel. For more photos and information, see pages 8 and 9. Training Wing changes command Colonel Arturo Di Martino assumes command of the Training Wing on Sept. 20, 2013. Photo by Wiel Borghans By Maureen Geraets-Head The new Training Wing Commander, Colonel Di Martino said in his The Trapani Room at the E-3A Club speech, “I am fully aware of the huge is filled with guests attending the responsibilities that are connected with Training Wing Change of Command. the fulfillment of a Wing Commander’s duties. Being in charge as the Wing With the marching in of the Colors Commander certainly means to be the ceremony starts. Colonel Arturo accountable. Accountable, not only to Di Martino, ITAAF,60¡ officially65¡ assumes70¡ the Component75¡ 80¡ Commander,85¡ not only90¡ 95¡ 100¡ 105¡ 110¡ 115¡ 120¡ 125¡ 40¡ command of theUZBEKIS TrainingTAN Wing on to the flag that I’m representing in this KAZAKHST AN Sept. 20, 2013, after that Lt. Col. multinational environment,Dzunga but alsor ia to MONGOLIA Almaty Knud Holmsgaard has relinquished theBishkek other Wing Commanders†rŸmqi by giving GOBI Beijing Dalian Bukhara Oz. Issyk Kul' N command. Tashkent them the support and outputH thatA UZBEKISTAN KYRGYZSTAN S Hami Baotou Tianjin Bo Hai N Samarkand E they are expectingI from one’s unitT urinfan a Depression Aksu TURKMENISTAN T 35¡ Maj.Mashhad Gen. Andrew Mueller presided timely, proper manner, always keeping ORDOS Shijiazhuang 35¡ Dushanbe Qingdao over the change of command. -

A Historical and Linguistic Study of the German Settlement at Roberts Cove, Louisiana
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 A Historical and Linguistic Study of the German Settlement at Roberts Cove, Louisiana. Stanley Joe Mccord Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Mccord, Stanley Joe, "A Historical and Linguistic Study of the German Settlement at Roberts Cove, Louisiana." (1969). LSU Historical Dissertations and Theses. 1606. https://digitalcommons.lsu.edu/gradschool_disstheses/1606 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 70-253 McCORD, Stanley Joe, 1936- A HISTORICAL AND LINGUISTIC STUDY OF THE GERMAN SETTLEMENT AT ROBERTS COVE, LOUISIANA. [Portions of Text in German]. The Louisiana State University and Agricultural and Mechanical College, Ph.D., 1969 Language and Literature, modem University Microfilms, Inc., Ann Arbor, Michigan Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. A HISTORICAL AND LINGUISTIC STUDY OF THE GERMAN SETTLEMENT AT ROBERTS COVE, LOUISIANA A Dissertation Submitted, to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Foreign Languages fcy Stanley Joe McCord B,A.f Louisiana State University, i960 M.A., Louisiana State University, 1963 May, 1969 Reproduced with permission of the copyright owner. -

Multibus (Germany)
MultiBus (Germany) Name/ concept Multibus/ call-a-bus service Start date/ December 2003/ ongoing Duration City, region, country Municipalities Gangelt, Selfkant and Waldfeucht (all district Heinsberg), Germany Geographical area Region characterised by disperse settlements with approx. 30,000 inhabitants/ covered/ trip length medium trip length focus Modal focus/ mode Flexible bus service, aiming at door to door service (currently still stops in a max. integration distance of 200m to home, but drop off directly at destination) and connection to main public transport routes Financing/ funding Costs per year approx. 440,000 € Cost savings compared to traditional bus services 41,500 €/ year Research project within the programme “Public Transport for the Region” Human resources - Functionalities/ Reservation of service at the latest 30 min before pick up services/ facilities/ Includes a parcel delivery service to achieve additional revenues and to support vehicles marketing 4 low-floor Minibuses, using biodiesel Call centre Relevant Local public transport services (West Energie und Verkehr GmbH), Wuppertal stakeholders Institute for Climate, Environment and Energy as well as Harloff Hensel Stadtplanung Ingenieurgesellschaft GmbH (Consulting Engineer) Market segment & A target group survey at the beginning of the project showed that the core user user groups group would be children, teenagers, families and seniors targeted In 2004 approx. 2,900 passengers used the service each month Low demand during weekend Marketing Strategy for social marketing -

16-11-10 Team39 Tagestruktur-HS.Indd
Krefeld Standorte Tagesstruktur ViaNobis - Wir sind in ihrer Nähe. Kreis Heinsberg Die Eingliederungshilfe Viersen Niederheid Von-Siemens-Straße 6 Mönchengladbach 52511 Geilenkirchen Tel.: +49 2451 9434-714 Rheydt Fax: +49 2451 9434-713 [email protected] Tagesstruktur Odenkirchen Übach-Palenberg Beratung, Begleitung und Unterstützung Erkelenz Aachener Straße 88 Kreis Heinsberg Heinsberg 52531 Übach-Palenberg Hückelhoven Tel.: +49 2451 4906396 [email protected] Gangelt Kolpinghaus Gangelt Niederheid Wallstraße 23 52538 Gangelt Tel.: +49 2454 9389-815 Übach-Palenberg Fax: +49 2454 9389-820 [email protected] Sie fi nden uns hier: Heinsberg Erkelenz Valkenburger Straße 15 Gangelt 52525 Heinsberg Heinsberg Tel.: +49 2452 9778740 Hückelhoven Fax: +49 2452 8609605 Krefeld [email protected] Mönchengladbach Mönchengladbach-Odenkirchen Hückelhoven Niederheid Rheydt Martin-Luther-Straße 5a Übach-Palenberg 41836 Hückelhoven Viersen Tel.: +49 2433 9047817 Fax: +49 2433 9047819 TagesstrukturHü[email protected] Kontakt Erkelenz ViaNobis - Die Eingliederungshilfe Aachener Straße 30 41812 Erkelenz Bruchstraße 6 | 52538 Gangelt Tel.: +49 2431 9451516 Telefon: +49 2454 59-413 | Telefax: +49 2454 59-415 Fax: +49 2431 9452882 www.vianobis.de [email protected] Was heißt Tagesstruktur? Zielsetzung der Tagesstruktur Unsere Angebote in der Tagesstruktur Jeder Mensch braucht eine Tagesstruktur. Die Gestaltung des In einem geschützten und abstinenten Raum erfahren unsere Kreativität Papier- und Malarbeiten Tages in wiederkehrende, verlässliche Tages- und Wochen- Teilnehmer eine individuelle Förderung, Akzeptanz und Wert- ■ Holzarbeiten abläufe bietet Sicherheit und die Basis für die Förderung und schätzung. ■ Tonarbeiten den Erhalt von Fähigkeiten und Fertigkeiten. Für Menschen mit ■ Seidenmalerei Behinderungen ist sie als zweiter Lebensraum, ergänzend zum Hier können sie soziale Kontakte aufbauen, einer sinnhaften ■ Wohnbereich, von großer Bedeutung. -

Thriving in Geilenkirchen and Niederheid
Thriving In Geilenkirchen and Niederheid 1 Table of Contents Welcome .................................................................................................................................... 5 Contact Us ............................................................................................................................. 5 Staff Directory ........................................................................................................................ 6 Using the Phone ........................................................................................................................ 7 Emergency Numbers ............................................................................................................. 7 Frequently Called Numbers .................................................................................................... 8 Base Details ............................................................................................................................... 9 CFSU(E) Niederheid .............................................................................................................. 9 NATO Air Base Geilenkirchen ...............................................................................................11 JFC Brunssum ......................................................................................................................13 Languages ................................................................................................................................15 Holidays -

Coronavirus: Virologist Believes That Infection Through Surface Contact Is Unlikely
Coronavirus: Virologist believes that infection through surface contact is unlikely Author: Volker Blasek, editor, certified professional Source: University Hospital Bonn, North Rhine-Westphalia, Coronavirus Research Project COVID-19 in collaboration with the Institute of Virology at the University Hospital Bonn (published March 27, 2020), ukbnewsroom.de April 7, 2020 The text is drawn up in accordance with scientific standards and checked by medical professionals* *This text complies with published medical articles, guidelines, and research results and has been checked by medical professionals. What is the risk of infection with SARS-CoV-2 (COVID-19) by way of contact with surfaces? Controversial opinions concerning the risk of infection with the new SARS-CoV-2 coronavirus (COVID-19) by way of contact with infected surfaces currently exist. Some laboratory studies have shown that the virus can survive on certain surfaces. But does the same thing happen in real-world conditions? Based on his research, virologist Professor Hendrik Streeck believes that the risk of infection by way of surface contact is extremely low . Professor Hendrick Streeck, together with Professor Dr. Christian Drosten, is currently perhaps the most famous virologist in Germany. A young lead researcher has taken over Drosten as director of the Institute of Virology at the University Hospital in Bonn. He and his team use a slightly different approach to work. While many virologists mainly work in laboratories, the Streek team collects data in the vicinity of Heinsberg, which is a high-risk area. And they have already received important unexpected results. Has the risk of infection through surface contact been overestimated? It has now been scientifically proven that SARS-CoV-2 (COVID-19) can be spread by airborne droplets. -
Uebersichten Netzbetreiber NEW-Netz 120315.Indd
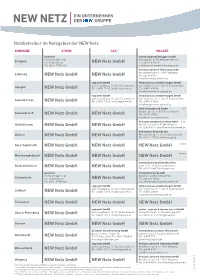
Netzbetreiber im Netzgebiet der NEW Netz KOMMUNE STROM GAS * WASSER westnetz Gemeindewerke Brüggen GmbH Florianstraße 15-21 Holtweg 60, 41379 Brüggen-Bracht Brüggen 44139 Dortmund NEW Netz GmbH Tel. 02157 873670 Tel. 0800 93786389 [email protected] Kreiswasserwerk Heinsberg GmbH Am Wasserwerk 5, 41844 Wegberg Erkelenz NEW Netz GmbH NEW Netz GmbH Tel. 02434 8070 [email protected] regionetz GmbH Verbandswasserwerk Gangelt GmbH Zum Hagelkreuz 16, 52249 Eschweiler Von-Siemens Str. 4, 52511 Geilenkirchen Gangelt NEW Netz GmbH Tel. 02403 701-0, [email protected] Tel. 02451 49008 [email protected] regionetz GmbH Verbandswasserwerk Gangelt GmbH Zum Hagelkreuz 16, 52249 Eschweiler Von-Siemens Str. 4, 52511 Geilenkirchen Geilenkirchen NEW Netz GmbH Tel. 02403 701-0, [email protected] Tel. 02451 49008 [email protected] GWG Grevenbroich GmbH Nordstraße 36, 41515 Grevenbroich ** Grevenbroich NEW Netz GmbH NEW Netz GmbH Tel. 02181 65050 [email protected] Kreiswasserwerk Heinsberg GmbH Hückelhoven NEW Netz GmbH NEW Netz GmbH Am Wasserwerk 5, 41844 Wegberg *** Tel. 02434 8070, [email protected] Kreiswerke Grevenbroich Jüchen NEW Netz GmbH NEW Netz GmbH Am Schellberg 14, 41516 Grevenbroich Tel. 02182 1705-0, [email protected] Korschenbroich NEW Netz GmbH NEW Netz GmbH NEW Netz GmbH **** Mönchengladbach NEW Netz GmbH NEW Netz GmbH NEW Netz GmbH ***** Gemeindewerke Niederkrüchten Niederkrüchten NEW Netz GmbH NEW Netz GmbH Dam 107, 41372 Niederkrüchten Tel. 02163 98310, [email protected] westnetz Schwalmtalwerke AöR Florianstraße 15-21 Markt 20, 41366 Schwalmtal Schwalmtal 44139 Dortmund NEW Netz GmbH Tel. 02163 946132 Tel. 0800 93786389 [email protected] regionetz GmbH Verbandswasserwerk Gangelt GmbH Zum Hagelkreuz 16, 52249 Eschweiler Von-Siemens Str. -

Downloads to Common Statistical Packages; and 4) Procedures for Data Integration and Interoperability with External Sources
medRxiv preprint doi: https://doi.org/10.1101/2020.05.04.20090076; this version posted May 8, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Infection fatality rate of SARS-CoV-2 infection in a German community with a super-spreading event Hendrik Streeck1, Bianca Schulte1, Beate M. Kümmerer1, Enrico Richter1, Tobias Höller5, Christine Fuhrmann5, Eva Bartok4, Ramona Dolscheid4, Moritz Berger3, Lukas WessendorF1, Monika Eschbach-Bludau1, Angelika Kellings5, Astrid Schwaiger6, Martin Coenen5, Per HoFFmann7, Birgit StoFFel-Wagner4, Markus M. Nöthen7, Anna-Maria Eis-Hübinger1, Martin Exner2, Ricarda Maria Schmithausen2, Matthias Schmid3 and Gunther Hartmann4 1 Institute of Virology, University Hospital, University oF Bonn, Germany, and German Center for Infection Research (DZIF), partner site Bonn-Cologne 2 Institute for Hygiene and Public Health, University Hospital, University oF Bonn, Germany 3 Institute for Medical Biometry, Informatics and Epidemiology, University Hospital, University of Bonn, Germany 4 Institute of Clinical Chemistry and Clinical Pharmacology, University Hospital, University oF Bonn, Germany; German Center For InFection Research (DZIF), partner site Bonn-Cologne 5 Clinical Study Core Unit, Study Center Bonn (SZB), Institute of Clinical Chemistry and Clinical Pharmacology, University Hospital, University oF Bonn, Germany 6 Biobank -

COVID-19: First Long-Term Care Facility Outbreak in the Netherlands Following Cross-Border Introduction from Germany, March 2020 Mitch Van Hensbergen1,2* , Casper D
van Hensbergen et al. BMC Infectious Diseases (2021) 21:418 https://doi.org/10.1186/s12879-021-06093-9 RESEARCH ARTICLE Open Access COVID-19: first long-term care facility outbreak in the Netherlands following cross-border introduction from Germany, March 2020 Mitch van Hensbergen1,2* , Casper D. J. den Heijer1,2,3, Petra Wolffs3, Volker Hackert1, Henriëtte L. G. ter Waarbeek1, Bas B. Oude Munnink4, Reina S. Sikkema4, Edou R. Heddema5 and Christian J. P. A. Hoebe1,2,3 Abstract Background: The Dutch province of Limburg borders the German district of Heinsberg, which had a large cluster of COVID-19 cases linked to local carnival activities before any cases were reported in the Netherlands. However, Heinsberg was not included as an area reporting local or community transmission per the national case definition at the time. In early March, two residents from a long-term care facility (LTCF) in Sittard, a Dutch town located in close vicinity to the district of Heinsberg, tested positive for COVID-19. In this study we aimed to determine whether cross-border introduction of the virus took place by analysing the LTCF outbreak in Sittard, both epidemiologically and microbiologically. Methods: Surveys and semi-structured oral interviews were conducted with all present LTCF residents by health care workers during regular points of care for information on new or unusual signs and symptoms of disease. Both throat and nasopharyngeal swabs were taken from residents suspect of COVID-19, based on regional criteria, for the detection of SARS-CoV-2 by Real-time Polymerase Chain Reaction. Additionally, whole genome sequencing was performed using a SARS-CoV-2 specific amplicon-based Nanopore sequencing approach. -

DUK Donates Checks for Charity in Local Community NATO AWACS
29 March 2016 NATO Skywatch 1 Volume 32, No. 3 • 29 March 2016 NATO AWACS Assurance to Turkey Photo by Staff Sgt. Alexandra Longfellow NATO AWACS in Konya, Turkey. Following a recent increase in cross-border activities with Russia, Turkey requested additional military support to the region. This additional support, approved by NATO, comes in the form of Tailored Assurance Measures and includes an increase in AWACS presence in the region. DUK donates checks for charity in local community Story by Alex Gieswein and Carsten Chairman, handed over checks to nine keep it as familiar as possible,” Alex started. On Sunday, the family day, we Brueggenolte organizations. Gieswein said. plan to entertain you with a magician, Photo by Katharina Krämer a bouncing castle, face-painting and “We do not want to keep the profits Institutions and associations that an E-3A aircraft with “Static-Display” The E-3A Component Oktoberfest is for us alone, we want to donate it have projects in the pipeline next year to show you how the people work one of the most anticipated events to the local community” said Brig. can apply for donations. The planning when they are in the air. And many at NATO Air Base Geilenkirchen, Gen. Karsten Stoye, and MSgt Alex for the next Oktoberfest has already more! Germany. Every year, several Gieswein added that “With this money thousand people attend the we can support projects which would event, which is organized by the not be possible otherwise”. This year German non-commissioned officer the donations went to the carnival camaraderie (DUK).