Tracing the Origin of Planktonic Protists in an Ancient Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -
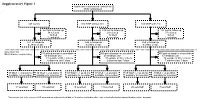
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Why the –Omic Future of Apicomplexa Should Include Gregarines Julie Boisard, Isabelle Florent
Why the –omic future of Apicomplexa should include Gregarines Julie Boisard, Isabelle Florent To cite this version: Julie Boisard, Isabelle Florent. Why the –omic future of Apicomplexa should include Gregarines. Biology of the Cell, Wiley, 2020, 10.1111/boc.202000006. hal-02553206 HAL Id: hal-02553206 https://hal.archives-ouvertes.fr/hal-02553206 Submitted on 24 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Article title: Why the –omic future of Apicomplexa should include Gregarines. Names of authors: Julie BOISARD1,2 and Isabelle FLORENT1 Authors affiliations: 1. Molécules de Communication et Adaptation des Microorganismes (MCAM, UMR 7245), Département Adaptations du Vivant (AVIV), Muséum National d’Histoire Naturelle, CNRS, CP52, 57 rue Cuvier 75231 Paris Cedex 05, France. 2. Structure et instabilité des génomes (STRING UMR 7196 CNRS / INSERM U1154), Département Adaptations du vivant (AVIV), Muséum National d'Histoire Naturelle, CP 26, 57 rue Cuvier 75231 Paris Cedex 05, France. Short Title: Gregarines –omics for Apicomplexa studies -

Tracing the Origin of Planktonic Protists in an Ancient Lake
microorganisms Article Tracing the Origin of Planktonic Protists in an Ancient Lake Nataliia V. Annenkova 1,* , Caterina R. Giner 2,3 and Ramiro Logares 2,* 1 Limnological Institute Siberian Branch of the Russian Academy of Sciences 3, Ulan-Batorskaya St., 664033 Irkutsk, Russia 2 Institute of Marine Sciences (ICM), CSIC, Passeig Marítim de la Barceloneta, 37-49, ES08003 Barcelona, Spain; [email protected] 3 Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver, BC V6T 1Z4, Canada * Correspondence: [email protected] (N.V.A.); [email protected] (R.L.) Received: 26 February 2020; Accepted: 7 April 2020; Published: 9 April 2020 Abstract: Ancient lakes are among the most interesting models for evolution studies because their biodiversity is the result of a complex combination of migration and speciation. Here, we investigate the origin of single celled planktonic eukaryotes from the oldest lake in the world—Lake Baikal (Russia). By using 18S rDNA metabarcoding, we recovered 1414 Operational Taxonomic Units (OTUs) belonging to protists populating surface waters (1–50 m) and representing pico/nano-sized cells. The recovered communities resembled other lacustrine freshwater assemblages found elsewhere, especially the taxonomically unclassified protists. However, our results suggest that a fraction of Baikal protists could belong to glacial relicts and have close relationships with marine/brackish species. Moreover, our results suggest that rapid radiation may have occurred among some protist taxa, partially mirroring what was already shown for multicellular organisms in Lake Baikal. We found 16% of the OTUs belonging to potential species flocks in Stramenopiles, Alveolata, Opisthokonta, Archaeplastida, Rhizaria, and Hacrobia. -

Assessing the Efficiency of Molecular Markers for the Species Identification of Gregarines Isolated from the Mealworm and Super Worm Midgut
Microorganisms 2018, 6, 119 1 of 20 Article Assessing the Efficiency of Molecular Markers for the Species Identification of Gregarines Isolated from the Mealworm and Super Worm Midgut Chiara Nocciolini 1, Claudio Cucini 2, Chiara Leo 2, Valeria Francardi 3, Elena Dreassi 4 and Antonio Carapelli 2,* 1 Polo d’Innovazione di Genomica Genetica e Biologia, 53100 Siena, Italy; [email protected] 2 Department of Life Sciences, University of Siena, 53100 Siena, Italy; [email protected] (C.C.); [email protected] (C.L.) 3 Consiglio per la ricerca in agricoltura e analisi dell’economia agraria – Centro di Difesa e Certificazione CREA-DC) Research Centre for Plant Protection and Certification, via di Lanciola 12/A, 50125 Firenze, Italy; [email protected] 4 Department of Biotechnology, Chemistry and Pharmacy, University of Siena, 53100 Siena, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0577-234-410 Received: 28 September 2018; Accepted: 23 November 2018; Published: 27 November 2018 Abstract: Protozoa, of the taxon Gregarinasina, are a heterogeneous group of Apicomplexa that includes ~1600 species. They are parasites of a large variety of both marine and terrestrial invertebrates, mainly annelids, arthropods and mollusks. Unlike coccidians and heamosporidians, gregarines have not proven to have a negative effect on human welfare; thus, they have been poorly investigated. This study focuses on the molecular identification and phylogeny of the gregarine species found in the midgut of two insect species that are considered as an alternative source of animal proteins for the human diet: the mealworm Tenebrio molitor, and the super-worm Zophobas atratus (Coleoptera: Tenebrionidae). -

Gregarines (Apicomplexa: Gregarinasina) Are Monoxenous Parasites of Invertebrates
Gregarines (Apicomplexa: Gregarinasina) are monoxenous parasites of invertebrates. Those found in sand flies (Diptera: Psychodidae) and mosquitoes (Diptera: Culicidae) used to be considered a single eugregarine genus Ascogregarina. Our phylogenetic analyses of the gregarine SSU rDNA, including newly obtained sequences of three species from sand flies, showed that mosquito and sand fly gregarines are closely related to neogregarines, and most importantly, they form two disparate monophyletic groups. Based on these molecular features, accompanied by biological differences, we established a new genus Psychodiella for the gregarines from sand flies, reserving the genus Ascogregarina for the mosquito gregarines. In the new genus, two new species Psychodiella sergenti from Phlebotomus sergenti and Psychodiella tobbi from Phlebotomus tobbi were described. They differ in the life cycles (sexual development of Ps. sergenti is triggered by a blood meal intake) and morphology of their life stages, mainly oocysts. The susceptibility of five sand fly species to both gregarines showed their strict host specificity, as they were able to fully develop and complete the life cycle only in their natural hosts. The life cycle of Ps. sergenti was studied in detail using various microscopical methods. Oocysts are attached to the chorion of sand fly eggs. Sporozoites, with a three- layered pellicle and mucron, attach to the 1st instar larval intestine but are never located intracellularly. In the 4th instar larvae, the gregarines occur in the ectoperitrophic space and later in the intestinal lumen. In adults, the parasites appear in the body cavity, and the sexual development of Ps. sergenti takes place only in blood-fed females; gametocysts attach to the accessory glands and oocysts are injected into their lumen. -

Phylogeny of ‘Araphid’ Diatoms Inferred from SSU and LSU Rdna, RBCL and PSBA Sequences Linda K
Phylogeny of ‘araphid’ diatoms inferred from SSU and LSU rDNA, RBCL and PSBA sequences Linda K. Medlin, Yves Desdevises To cite this version: Linda K. Medlin, Yves Desdevises. Phylogeny of ‘araphid’ diatoms inferred from SSU and LSU rDNA, RBCL and PSBA sequences. Vie et Milieu / Life & Environment, Observatoire Océanologique - Laboratoire Arago, 2016. hal-02144540 HAL Id: hal-02144540 https://hal.archives-ouvertes.fr/hal-02144540 Submitted on 26 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. VIE ET MILIEU - LIFE AND ENVIRONMENT, 2016, 66 (2): 129-154 PHYLOGENY OF ‘arapHID’ DIatoms INFErrED From SSU and LSU RDNA, RBCL AND PSBA SEQUENCES L. K. MEDLIN 1*, Y. DESDEVISES 2 1 Marine Biological Association of the UK, the Citadel, Plymouth, PL1 2PB UK Royal Botanic Gardens, Edinburgh, Scotland, UK 2 Sorbonne Universités, UPMC Univ Paris 06, CNRS, Biologie Intégrative des Organismes Marins (BIOM), Observatoire Océanologique, F-66650, Banyuls/Mer, France * Corresponding author: [email protected] ARAPHID ABSTRACT. – Phylogenies of the diatoms have largely been inferred from SSU rDNA sequenc- DIATOMS DIVERGENCE TIME ESTIMATION es. Because previously published SSU rDNA topologies of araphid pennate diatoms have var- LSU ied, a supertree was constructed in order to summarize those trees and used to guide further PINNATE analyses where problems arose. -

Proposal for Practical Multi-Kingdom Classification of Eukaryotes Based on Monophyly 2 and Comparable Divergence Time Criteria
bioRxiv preprint doi: https://doi.org/10.1101/240929; this version posted December 29, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Proposal for practical multi-kingdom classification of eukaryotes based on monophyly 2 and comparable divergence time criteria 3 Leho Tedersoo 4 Natural History Museum, University of Tartu, 14a Ravila, 50411 Tartu, Estonia 5 Contact: email: [email protected], tel: +372 56654986, twitter: @tedersoo 6 7 Key words: Taxonomy, Eukaryotes, subdomain, phylum, phylogenetic classification, 8 monophyletic groups, divergence time 9 Summary 10 Much of the ecological, taxonomic and biodiversity research relies on understanding of 11 phylogenetic relationships among organisms. There are multiple available classification 12 systems that all suffer from differences in naming, incompleteness, presence of multiple non- 13 monophyletic entities and poor correspondence of divergence times. These issues render 14 taxonomic comparisons across the main groups of eukaryotes and all life in general difficult 15 at best. By using the monophyly criterion, roughly comparable time of divergence and 16 information from multiple phylogenetic reconstructions, I propose an alternative 17 classification system for the domain Eukarya to improve hierarchical taxonomical 18 comparability for animals, plants, fungi and multiple protist groups. Following this rationale, 19 I propose 32 kingdoms of eukaryotes that are treated in 10 subdomains. These kingdoms are 20 further separated into 43, 115, 140 and 353 taxa at the level of subkingdom, phylum, 21 subphylum and class, respectively (http://dx.doi.org/10.15156/BIO/587483). -

Molecular Tools for the Study of Marine Microbial Diversity - L.K
BIOTECHNOLOGY –Vol. IX - Molecular Tools for the Study of Marine Microbial Diversity - L.K. Medlin, K. Valentin, K. Metfies, K. Töbe, R. Groben MOLECULAR TOOLS FOR THE STUDY OF MARINE MICROBIAL DIVERSITY L.K. Medlin, K. Valentin, K. Metfies and K. Töbe Department of Biological Oceanography, Alfred Wegener Institute for Polar and Marine Research, Germany R. Groben Lake Ecosystem Group, Centre for Ecology & Hydrology, United Kingdom Keywords: AFLP, algae, biodiversity, clone library, DGGE, DNA fingerprinting, DNA microarrays, FISH, Fluorescence In Situ Hybridization, microsatellites, molecular marker, molecular probes, oligonucleotide probes, PCR, phylogeny, phytoplankton, RAPD, RFLP, rRNA, solid-phase cytometry, SSCP, TGGE. Contents 1. The Importance of Biodiversity Research in the Marine Environment 2. What Questions can be Answered Using Molecular Biology Techniques? 3. Evaluating Marine Biodiversity by Sequence Analysis and Fingerprinting Methods 3.1. Sequence Analysis 3.1.1. Which Genes to Select? 3.1.2. How to Generate Sequence Data? 3.1.3. Determining Biodiversity in an Environmental Sample by Sequence Analysis 3.1.4. Analysing Sequences for Determining Phylogenies and Biodiversity 3.1.5. DNA Barcoding 3.2. Fingerprinting Methods 4. Analysis of Population Structure Using Molecular Markers 5. Molecular Probes for Identification and Characterisation of Marine Phytoplankton 5.1. Introduction 5.2. Probe Design 5.3. Detection Methods 6. Conclusions Acknowledgements Glossary UNESCO – EOLSS Bibliography Biographical Sketches Summary SAMPLE CHAPTERS Marine photosynthetic microbial organisms are the major, sustaining components of ecosystem processes and are responsible for biogeochemical reactions that drive our climate changes. Despite this, many marine microorganisms are poorly described and little is known of broad spatial and temporal scale trends in their abundance and distribution. -

From Freshwater Fishes in Africa (Tomáš Scholz)
0 Organizer: Department of Botany and Zoology, Faculty of Science, Masaryk University, Kotlářská 2, 611 37 Brno, Czech Republic Workshop venue: Instutute of Vertebrate Biology, Academy of Sciences CR Workshop date: 28 November 2018 Cover photo: Research on fish parasites throughout Africa: Fish collection in, Lake Turkana, Kenya; Fish examination in the Sudan; Teaching course on fish parasitology at the University of Khartoum, Sudan; Field laboratory in the Sudan Authors of cover photo: R. Blažek, A. de Chambrier and R. Kuchta All rights reserved. No part of this e-book may be reproduced or transmitted in any form or by any means without prior written permission of copyright administrator which can be contacted at Masaryk University Press, Žerotínovo náměstí 9, 601 77 Brno. © 2018 Masaryk University The stylistic revision of the publication has not been performed. The authors are fully responsible for the content correctness and layout of their contributions. ISBN 978-80-210-9079-8 ISBN 978-80-210-9083-5 (online: pdf) 1 Contents (We present only the first author in contents) ECIP Scientific Board ....................................................................................................................... 5 List of attendants ............................................................................................................................ 6 Programme ..................................................................................................................................... 7 Abstracts ........................................................................................................................................ -

Haemocystidium Spp., a Species Complex Infecting Ancient Aquatic
IDENTIFICACIÓN DE HEMOPARÁSITOS PRESENTES EN LA HERPETOFAUNA DE DIFERENTES DEPARTAMENTOS DE COLOMBIA. Leydy Paola González Camacho Universidad Nacional de Colombia Facultad de ciencias, Instituto de Biotecnología IBUN Bogotá, Colombia 2019 IDENTIFICACIÓN DE HEMOPARÁSITOS PRESENTES EN LA HERPETOFAUNA DE DIFERENTES DEPARTAMENTOS DE COLOMBIA. Leydy Paola González Camacho Tesis o trabajo de investigación presentada(o) como requisito parcial para optar al título de: Magister en Microbiología. Director (a): Ph.D MSc Nubia Estela Matta Camacho Codirector (a): Ph.D MSc Mario Vargas-Ramírez Línea de Investigación: Biología molecular de agentes infecciosos Grupo de Investigación: Caracterización inmunológica y genética Universidad Nacional de Colombia Facultad de ciencias, Instituto de biotecnología (IBUN) Bogotá, Colombia 2019 IV IDENTIFICACIÓN DE HEMOPARÁSITOS PRESENTES EN LA HERPETOFAUNA DE DIFERENTES DEPARTAMENTOS DE COLOMBIA. A mis padres, A mi familia, A mi hijo, inspiración en mi vida Agradecimientos Quiero agradecer especialmente a mis padres por su contribución en tiempo y recursos, así como su apoyo incondicional para la culminación de este proyecto. A mi hijo, Santiago Suárez, quien desde que llego a mi vida es mi mayor inspiración, y con quien hemos demostrado que todo lo podemos lograr; a Juan Suárez, quien me apoya, acompaña y no me ha dejado desfallecer, en este logro. A la Universidad Nacional de Colombia, departamento de biología y el posgrado en microbiología, por permitirme formarme profesionalmente; a Socorro Prieto, por su apoyo incondicional. Doy agradecimiento especial a mis tutores, la profesora Nubia Estela Matta y el profesor Mario Vargas-Ramírez, por el apoyo en el desarrollo de esta investigación, por su consejo y ayuda significativa con esta investigación. -

Symbiomonas Scintillans Gen. Et Sp. Nov. and Picophagus Flagellatus Gen
Protist, Vol. 150, 383–398, December 1999 © Urban & Fischer Verlag http://www.urbanfischer.de/journals/protist Protist ORIGINAL PAPER Symbiomonas scintillans gen. et sp. nov. and Picophagus flagellatus gen. et sp. nov. (Heterokonta): Two New Heterotrophic Flagellates of Picoplanktonic Size Laure Guilloua, 1, 2, Marie-Josèphe Chrétiennot-Dinetb, Sandrine Boulbena, Seung Yeo Moon-van der Staaya, 3, and Daniel Vaulota a Station Biologique, CNRS, INSU et Université Pierre et Marie Curie, BP 74, F-29682 Roscoff Cx, France b Laboratoire d’Océanographie biologique, UMR 7621 CNRS/INSU/UPMC, Laboratoire Arago, O.O.B., B.P. 44, F-66651 Banyuls sur mer Cx, France Submitted July 27, 1999; Accepted November 10, 1999 Monitoring Editor: Michael Melkonian Two new oceanic free-living heterotrophic Heterokonta species with picoplanktonic size (< 2 µm) are described. Symbiomonas scintillans Guillou et Chrétiennot-Dinet gen. et sp. nov. was isolated from samples collected both in the equatorial Pacific Ocean and the Mediterranean Sea. This new species possesses ultrastructural features of the bicosoecids, such as the absence of a helix in the flagellar transitional region (found in Cafeteria roenbergensis and in a few bicosoecids), and a flagellar root system very similar to that of C. roenbergensis, Acronema sippewissettensis, and Bicosoeca maris. This new species is characterized by a single flagellum with mastigonemes, the presence of en- dosymbiotic bacteria located close to the nucleus, the absence of a lorica and a R3 root composed of a 6+3+x microtubular structure. Phylogenetical analyses of nuclear-encoded SSU rDNA gene se- quences indicate that this species is close to the bicosoecids C.