Assessing the Efficiency of Molecular Markers for the Species Identification of Gregarines Isolated from the Mealworm and Super Worm Midgut
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Aspects About Supella Longipalpa (Blattaria: Blattellidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Asian Pac J Trop Biomed 2016; 6(12): 1065–1075 1065 HOSTED BY Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Biomedicine journal homepage: www.elsevier.com/locate/apjtb Review article http://dx.doi.org/10.1016/j.apjtb.2016.08.017 New aspects about Supella longipalpa (Blattaria: Blattellidae) Hassan Nasirian* Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran ARTICLE INFO ABSTRACT Article history: The brown-banded cockroach, Supella longipalpa (Blattaria: Blattellidae) (S. longipalpa), Received 16 Jun 2015 recently has infested the buildings and hospitals in wide areas of Iran, and this review was Received in revised form 3 Jul 2015, prepared to identify current knowledge and knowledge gaps about the brown-banded 2nd revised form 7 Jun, 3rd revised cockroach. Scientific reports and peer-reviewed papers concerning S. longipalpa and form 18 Jul 2016 relevant topics were collected and synthesized with the objective of learning more about Accepted 10 Aug 2016 health-related impacts and possible management of S. longipalpa in Iran. Like the Available online 15 Oct 2016 German cockroach, the brown-banded cockroach is a known vector for food-borne dis- eases and drug resistant bacteria, contaminated by infectious disease agents, involved in human intestinal parasites and is the intermediate host of Trichospirura leptostoma and Keywords: Moniliformis moniliformis. Because its habitat is widespread, distributed throughout Brown-banded cockroach different areas of homes and buildings, it is difficult to control. -

UNIVERSITY of CALIFORNIA RIVERSIDE Isolation
UNIVERSITY OF CALIFORNIA RIVERSIDE Isolation, Determination of Absolute Stereochemistry, and Asymmetric Synthesis of Insect Methyl-Branched Hydrocarbons A Dissertation submitted in the partial satisfaction of the requirements for the degree of Doctor of Philosophy in Chemistry by Jan Edgar Bello June 2014 Dissertation Committee: Dr. Jocelyn G. Millar, Chairperson Dr. Thomas H. Morton Dr. Catharine Larsen Copyright by Jan Edgar Bello 2014 The Dissertation of Jan Edgar Bello is approved: ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ Committee Chairperson University of California, Riverside Acknowledgements This dissertation would not have been possible without the guidance, assistance, and support from both my academic and biological families. I would first and foremost like to thank my advisor Professor Jocelyn G. Millar, who has guided me through this rigorous process and has helped me become the chemical ecologist I am today. I would also like to thank my research group Dr. Steve McElfresh, Dr. Yunfan Zou, Dr. Rebeccah Waterworth, R. Max Collignon, Joshua Rodstein, Jackie Serrano, and Brian Hanley for all the suggestions, insect collecting, synthetic discussions, and experimental advise that have allowed me to complete this dissertation. I would like to send a huge thank you to my family who have always believed in me. To my mom and dad, thank you for your encouragement, for loving me, and for your support (both financial and emotional). To my siblings, Jonathan, Michelle, and John-C thank you for your encouragement and for praying for me, especially during the beginning of my PhD studies when things were overwhelming. I would also like to thank my friends, Ryan Neff, Lauren George, Jenifer N. -

A New Insect Trackway from the Upper Jurassic—Lower Cretaceous Eolian Sandstones of São Paulo State, Brazil: Implications for Reconstructing Desert Paleoecology
A new insect trackway from the Upper Jurassic—Lower Cretaceous eolian sandstones of São Paulo State, Brazil: implications for reconstructing desert paleoecology Bernardo de C.P. e M. Peixoto1,2, M. Gabriela Mángano3, Nicholas J. Minter4, Luciana Bueno dos Reis Fernandes1 and Marcelo Adorna Fernandes1,2 1 Laboratório de Paleoicnologia e Paleoecologia, Departamento de Ecologia e Biologia Evolutiva, Universidade Federal de São Carlos (UFSCar), São Carlos, São Paulo, Brazil 2 Programa de Pós Graduacão¸ em Ecologia e Recursos Naturais, Centro de Ciências Biológicas e da Saúde, Universidade Federal de São Carlos (UFSCar), São Carlos, São Paulo, Brazil 3 Department of Geological Sciences, University of Saskatchewan, Saskatoon, Saskatchewan, Canada 4 School of the Environment, Geography, and Geosciences, University of Portsmouth, Portsmouth, Hampshire, United Kingdom ABSTRACT The new ichnospecies Paleohelcura araraquarensis isp. nov. is described from the Upper Jurassic-Lower Cretaceous Botucatu Formation of Brazil. This formation records a gigantic eolian sand sea (erg), formed under an arid climate in the south-central part of Gondwana. This trackway is composed of two track rows, whose internal width is less than one-quarter of the external width, with alternating to staggered series, consisting of three elliptical tracks that can vary from slightly elongated to tapered or circular. The trackways were found in yellowish/reddish sandstone in a quarry in the Araraquara municipality, São Paulo State. Comparisons with neoichnological studies and morphological inferences indicate that the producer of Paleohelcura araraquarensis isp. nov. was most likely a pterygote insect, and so could have fulfilled one of the Submitted 6 November 2019 ecological roles that different species of this group are capable of performing in dune Accepted 10 March 2020 deserts. -

ABSTRACT Gregarine Parasitism in Dragonfly Populations of Central
ABSTRACT Gregarine Parasitism in Dragonfly Populations of Central Texas with an Assessment of Fitness Costs in Erythemis simplicicollis Jason L. Locklin, Ph.D. Mentor: Darrell S. Vodopich, Ph.D. Dragonfly parasites are widespread and frequently include gregarines (Phylum Apicomplexa) in the gut of the host. Gregarines are ubiquitous protozoan parasites that infect arthropods worldwide. More than 1,600 gregarine species have been described, but only a small percentage of invertebrates have been surveyed for these apicomplexan parasites. Some consider gregarines rather harmless, but recent studies suggest otherwise. Odonate-gregarine studies have more commonly involved damselflies, and some have considered gregarines to rarely infect dragonflies. In this study, dragonfly populations were surveyed for gregarines and an assessment of fitness costs was made in a common and widespread host species, Erythemis simplicicollis. Adult dragonfly populations were surveyed weekly at two reservoirs in close proximity to one another and at a flow-through wetland system. Gregarine prevalences and intensities were compared within host populations between genders, among locations, among wing loads, and through time. Host fitness parameters measured included wing load, egg size, clutch size, and total egg count. Of the 37 dragonfly species surveyed, 14 species (38%) hosted gregarines. Thirteen of those species were previously unreported as hosts. Gregarine prevalences ranged from 2% – 52%. Intensities ranged from 1 – 201. Parasites were aggregated among their hosts. Gregarines were found only in individuals exceeding a minimum wing load, indicating that gregarines are likely not transferred from the naiad to adult during emergence. Prevalence and intensity exhibited strong seasonality during both years at one of the reservoirs, but no seasonal trend was detected at the wetland. -

Statistical Comparison of Excystation Methods in Cryptosporidium Parvum Oocysts
MASARYK UNIVERSITY FACULTY OF SCIENCE DEPT . OF BOTANY AND ZOOLOG Y BIOLOGICAL LY ACTIVE COMPOUNDS WIT H POTENTIAL ANTIPARASI TIC EFFECT AND THEIR IMPACT ON THE COURSE OF SELECTED PARASITOSES Ph.D. Dissertation Radka Pecková Supervisor: MVDr. Ivona Foitová, Ph.D . Brno 2018 Bibliographic Entry Author Mgr. Radka Pecková Faculty of Science, Masaryk University Department of Botany and Zoology Biological ly active compounds with potential Title of Thesis: antiparasitic effect and their impact on the processes of selected parasitoses Degree programme: Biology Field of Study: Parasitology Supervisor: MVDr. Ivona Foitová, Ph.D. Academic Year: 2017/2018 Number of Pages: 139 Keywords: Giardia intestinalis ; Cryptosporidium ; Anti - protozoal activity; Plant extracts; Drug of Choice; Natural Antiparasitics; Parasites; Archidendron fagifolium ; Diospyros sumatrana ; Piper betle ; Shorea sumatrana Bibliografický záznam Autor: Mgr. Radka Pecková Přírodovědecká fakulta, Masarykova univerzita Ústav botaniky a zoologie Biologicky aktivní látky s potencionálním Název práce: antiparazitárním účinkem a jejich působení na průběh vybraných parazitóz Studijní program: Biologie Studijní obor: Parazitologie Vedoucí práce: MVDr. Ivona Foitová, Ph.D. Akademický rok: 2017/2018 Počet stran: 139 Klíčová slova: Giardia intestinalis ; Cryptosporidium ; Antiprotozoární aktivita; Rostlinné extrakty; Alternativní léčiva; Přírodní antiparazitika; Paraziti; Archidendron fagifolium ; Diospyros sumatrana ; Piper betle ; Shorea sumatrana ABSTRAK T ABSTRACT This thesis deals with the study of the influence of extracts of selected plants from Indonesia on parasites Giardia intestinalis (Lambl) Alexeieff, 1914 and Cryptosporidium proliferans Kváč, Havrdová, Hlásková, Daňková, Kanděra, Ježková, Vítovec, Sak, Ortega, Xiao, Modrý, Jesudoss Chelladural, Prantlová & McEvoy, 2016 . Tested plants were selected based on behavioural data and the ability to reduce the intensity of parasitic infection in Sumatran orangutans. -
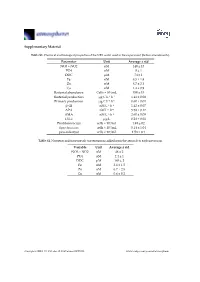
Supplementary Material Parameter Unit Average ± Std NO3 + NO2 Nm
Supplementary Material Table S1. Chemical and biological properties of the NRS water used in the experiment (before amendments). Parameter Unit Average ± std NO3 + NO2 nM 140 ± 13 PO4 nM 8 ± 1 DOC μM 74 ± 1 Fe nM 8.5 ± 1.8 Zn nM 8.7 ± 2.1 Cu nM 1.4 ± 0.9 Bacterial abundance Cells × 104/mL 350 ± 15 Bacterial production μg C L−1 h−1 1.41 ± 0.08 Primary production μg C L−1 h−1 0.60 ± 0.01 β-Gl nM L−1 h−1 1.42 ± 0.07 APA nM L−1 h−1 5.58 ± 0.17 AMA nM L−1·h−1 2.60 ± 0.09 Chl-a μg/L 0.28 ± 0.01 Prochlorococcus cells × 104/mL 1.49 ± 02 Synechococcus cells × 104/mL 5.14 ± 1.04 pico-eukaryot cells × 103/mL 1.58 × 0.1 Table S2. Nutrients and trace metals concentrations added from the aerosols to each mesocosm. Variable Unit Average ± std NO3 + NO2 nM 48 ± 2 PO4 nM 2.4 ± 1 DOC μM 165 ± 2 Fe nM 2.6 ± 1.5 Zn nM 6.7 ± 2.5 Cu nM 0.6 ± 0.2 Atmosphere 2019, 10, 358; doi:10.3390/atmos10070358 www.mdpi.com/journal/atmosphere Atmosphere 2019, 10, 358 2 of 6 Table S3. ANOVA test results between control, ‘UV-treated’ and ‘live-dust’ treatments at 20 h or 44 h, with significantly different values shown in bold. ANOVA df Sum Sq Mean Sq F Value p-value Chl-a 20 H 2, 6 0.03, 0.02 0.02, 0 4.52 0.0634 44 H 2, 6 0.02, 0 0.01, 0 23.13 0.002 Synechococcus Abundance 20 H 2, 7 8.23 × 107, 4.11 × 107 4.11 × 107, 4.51 × 107 0.91 0.4509 44 H 2, 7 5.31 × 108, 6.97 × 107 2.65 × 108, 1.16 × 107 22.84 0.0016 Prochlorococcus Abundance 20 H 2, 8 4.22 × 107, 2.11 × 107 2.11 × 107, 2.71 × 106 7.77 0.0216 44 H 2, 8 9.02 × 107, 1.47 × 107 4.51 × 107, 2.45 × 106 18.38 0.0028 Pico-eukaryote -
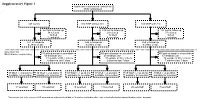
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Why the –Omic Future of Apicomplexa Should Include Gregarines Julie Boisard, Isabelle Florent
Why the –omic future of Apicomplexa should include Gregarines Julie Boisard, Isabelle Florent To cite this version: Julie Boisard, Isabelle Florent. Why the –omic future of Apicomplexa should include Gregarines. Biology of the Cell, Wiley, 2020, 10.1111/boc.202000006. hal-02553206 HAL Id: hal-02553206 https://hal.archives-ouvertes.fr/hal-02553206 Submitted on 24 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Article title: Why the –omic future of Apicomplexa should include Gregarines. Names of authors: Julie BOISARD1,2 and Isabelle FLORENT1 Authors affiliations: 1. Molécules de Communication et Adaptation des Microorganismes (MCAM, UMR 7245), Département Adaptations du Vivant (AVIV), Muséum National d’Histoire Naturelle, CNRS, CP52, 57 rue Cuvier 75231 Paris Cedex 05, France. 2. Structure et instabilité des génomes (STRING UMR 7196 CNRS / INSERM U1154), Département Adaptations du vivant (AVIV), Muséum National d'Histoire Naturelle, CP 26, 57 rue Cuvier 75231 Paris Cedex 05, France. Short Title: Gregarines –omics for Apicomplexa studies -

Social Context Modulates Idiosyncrasy of Behaviour in the Gregarious Cockroach Blaberus Discoidalis
bioRxiv preprint doi: https://doi.org/10.1101/028571; this version posted October 8, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Crall, Souffrant, Akandwanaho & Hescock, et al. 2015 – preprint version Social context modulates idiosyncrasy of behaviour in the gregarious cockroach Blaberus discoidalis James D. Crall1,2,*, André D. Souffrant1,*, Dominic Akandwanaho1,*, Sawyer D. Hescock1,*, Sarah E. Callan1,†, W. Melissa Coronado1,†, Maude W. Baldwin1, Benjamin L. de Bivort1,3,‡ 1 – Dept. of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA. 2 – Concord Field Station, Harvard University, Bedford, MA 01730, USA. 3 – Center for Brain Science, Harvard University, Cambridge, MA 02138, USA. * These authors contributed equally. † These authors contributed equally. ‡ Corresponding author: [email protected] Abstract Individuals are different, but they can work together to perform adaptive collective behaviours. Despite emerging evidence that individual variation strongly affects group performance, it is less clear to what extent individual variation is modulated by participation in collective behaviour. We examined light avoidance (negative phototaxis) in the gregarious cockroach Blaberus discoidalis, in both solitary and group contexts. Cockroaches in groups exhibit idiosyncratic light-avoidance performance that persists across days, with some individual cockroaches avoiding a light stimulus 75% of the time, and others avoiding the light just above chance (i.e. ~50% of the time). These individual differences are robust to group composition. Surprisingly, these differences do not persist when individuals are tested in isolation, but return when testing is once again done with groups. -

Tracing the Origin of Planktonic Protists in an Ancient Lake
microorganisms Article Tracing the Origin of Planktonic Protists in an Ancient Lake Nataliia V. Annenkova 1,* , Caterina R. Giner 2,3 and Ramiro Logares 2,* 1 Limnological Institute Siberian Branch of the Russian Academy of Sciences 3, Ulan-Batorskaya St., 664033 Irkutsk, Russia 2 Institute of Marine Sciences (ICM), CSIC, Passeig Marítim de la Barceloneta, 37-49, ES08003 Barcelona, Spain; [email protected] 3 Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver, BC V6T 1Z4, Canada * Correspondence: [email protected] (N.V.A.); [email protected] (R.L.) Received: 26 February 2020; Accepted: 7 April 2020; Published: 9 April 2020 Abstract: Ancient lakes are among the most interesting models for evolution studies because their biodiversity is the result of a complex combination of migration and speciation. Here, we investigate the origin of single celled planktonic eukaryotes from the oldest lake in the world—Lake Baikal (Russia). By using 18S rDNA metabarcoding, we recovered 1414 Operational Taxonomic Units (OTUs) belonging to protists populating surface waters (1–50 m) and representing pico/nano-sized cells. The recovered communities resembled other lacustrine freshwater assemblages found elsewhere, especially the taxonomically unclassified protists. However, our results suggest that a fraction of Baikal protists could belong to glacial relicts and have close relationships with marine/brackish species. Moreover, our results suggest that rapid radiation may have occurred among some protist taxa, partially mirroring what was already shown for multicellular organisms in Lake Baikal. We found 16% of the OTUs belonging to potential species flocks in Stramenopiles, Alveolata, Opisthokonta, Archaeplastida, Rhizaria, and Hacrobia. -

In the Cockroach, Blaberus Discoidalis
J Comp Physiol A (1998) 182: 23±33 Ó Springer-Verlag 1998 ORIGINAL PAPER J. T. Watson á R. E. Ritzmann Leg kinematics and muscle activity during treadmill running in the cockroach, Blaberus discoidalis : II. Fast running Accepted: 24 May 1997 Abstract We have combined kinematic and electromy- ogram (EMG) analysis of running Blaberus discoidalis to Introduction examine how middle and hind leg kinematics vary with Arthropod neuromuscular systems provide useful mod- running speed and how the fast depressor coxa (Df) and els in which to study problems involving control of lo- fast extensor tibia (FETi) motor neurons aect kine- comotion. The legs of various arthropods are matic parameters. In the range 2.5±10 Hz, B. discoidalis characterized by a pattern of few motor neurons sup- increases step frequency by altering the joint velocity plying each muscle, with each motor neuron having and by reducing the time required for the transition from markedly diverse properties. In all insects studied so far, ¯exion to extension. For both Df and FETi the timing of femoral depressor and tibial extensor muscles that are recruitment coincides with the maximal frequency seen used during stance are innervated by at most three axons for the respective slow motor neurons. Df is ®rst re- (Pearson and Iles 1971; Burrows and Hoyle 1972; Wil- cruited at the beginning of coxa-femur (CF) extension. son 1979). The triply innervated muscles receive a single FETi is recruited in the latter half of femur-tibia (FT) slow excitatory axon, a single fast excitatory axon, and a extension during stance. Single muscle potentials pro- common inhibitory axon shared with an undetermined duced by these fast motor neurons do not have pro- number of other muscles. -

Gregarines (Apicomplexa: Gregarinasina) Are Monoxenous Parasites of Invertebrates
Gregarines (Apicomplexa: Gregarinasina) are monoxenous parasites of invertebrates. Those found in sand flies (Diptera: Psychodidae) and mosquitoes (Diptera: Culicidae) used to be considered a single eugregarine genus Ascogregarina. Our phylogenetic analyses of the gregarine SSU rDNA, including newly obtained sequences of three species from sand flies, showed that mosquito and sand fly gregarines are closely related to neogregarines, and most importantly, they form two disparate monophyletic groups. Based on these molecular features, accompanied by biological differences, we established a new genus Psychodiella for the gregarines from sand flies, reserving the genus Ascogregarina for the mosquito gregarines. In the new genus, two new species Psychodiella sergenti from Phlebotomus sergenti and Psychodiella tobbi from Phlebotomus tobbi were described. They differ in the life cycles (sexual development of Ps. sergenti is triggered by a blood meal intake) and morphology of their life stages, mainly oocysts. The susceptibility of five sand fly species to both gregarines showed their strict host specificity, as they were able to fully develop and complete the life cycle only in their natural hosts. The life cycle of Ps. sergenti was studied in detail using various microscopical methods. Oocysts are attached to the chorion of sand fly eggs. Sporozoites, with a three- layered pellicle and mucron, attach to the 1st instar larval intestine but are never located intracellularly. In the 4th instar larvae, the gregarines occur in the ectoperitrophic space and later in the intestinal lumen. In adults, the parasites appear in the body cavity, and the sexual development of Ps. sergenti takes place only in blood-fed females; gametocysts attach to the accessory glands and oocysts are injected into their lumen.