Tracing the Origin of Planktonic Protists in an Ancient Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

A Survey of Selected Economic Plants Virgil S
Eastern Illinois University The Keep Masters Theses Student Theses & Publications 1981 A Survey of Selected Economic Plants Virgil S. Priebe Eastern Illinois University This research is a product of the graduate program in Botany at Eastern Illinois University. Find out more about the program. Recommended Citation Priebe, Virgil S., "A Survey of Selected Economic Plants" (1981). Masters Theses. 2998. https://thekeep.eiu.edu/theses/2998 This is brought to you for free and open access by the Student Theses & Publications at The Keep. It has been accepted for inclusion in Masters Theses by an authorized administrator of The Keep. For more information, please contact [email protected]. T 11 F:SIS H El"">RODUCTION CERTIFICATE TO: Graduate Degree Candidates who have written formal theses. SUBJECT: Permission to reproduce theses. The University Library is rece1vrng a number of requests from other institutions asking permission to reproduce disse rta tions for inclusion in their library holdings. Although no copyright laws are involved, we feel that professional courtesy demands that permission be obtained from the author before we allow theses to be copied. Please sign one of the following statements: Booth Library of Eastern Illinois University has my permission to l end my thesis to a reputable college or university for the purpose of copying it for inclusion in that institution's library or res e ar ch holdings• . �� /ft/ ff nfl. Author I respectfully request Booth Library of Eastern Illinois University not allow my thesis be reproduced because -----� ---------- ---··· ·--·· · ----------------------------------- Date Author m A Survey o f Selected Economic Plants (TITLE) BY Virgil S. -

Terrestrial Cryptogam Diversity Across Productivity Gradients of Southern
Distribution and diversity of terrestrial mosses, liverworts and lichens along productivity gradients of a southern boreal forest J.M. Kranabetter1, P. Williston2, and W.H. MacKenzie3 1British Columbia Ministry of Forests and Range Bag 6000, Smithers, B.C., Canada, V0J 2N0 Ph# (250) 847-6389; Fax# (250) 847-6353 [email protected] 2Gentian Botanical Research 4861 Nielsen Road, Smithers B.C., Canada, V0J 2N2 Ph# (250) 877-7702 [email protected] 3British Columbia Ministry of Forests and Range Bag 6000, Smithers, B.C., Canada, V0J 2N0 Ph# (250) 847-6388; Fax# (250) 847-6353 [email protected] Abstract Terrestrial cryptogams (mosses, liverworts and lichens) provide a useful suite of species for monitoring forests, especially when species distributions and diversity attributes are well defined from benchmark sites. To facilitate this, we surveyed contrasting plant associations of an upland boreal forest (in British Columbia, Canada) to explore the association of soil productivity with cryptogam distribution and diversity. Total terrestrial cryptogam richness of the study sites (19 plots of 0.15 ha each) was 148 taxa, with 47 mosses, 23 liverworts, and 78 lichens. Soil productivity was strongly related to the distribution of cryptogams by guild (i.e. lichen species richness declined with productivity, while moss and liverwort richness increased) and substrate (i.e. species richness on forest floor and cobbles declined with productivity as species richness increased on coarse woody debris). Generalists comprised only 15% of the cryptogam community by species, and mesotrophic sites lacked some of the species adapted to the dry-poor or moist-rich ends of the producitivity spectrum. -

Diversity of Microorganisms in Biocrusts Surrounding Highly Saline Potash Tailing Piles in Germany
microorganisms Communication Diversity of Microorganisms in Biocrusts Surrounding Highly Saline Potash Tailing Piles in Germany Ekaterina Pushkareva 1,2,* , Veronika Sommer 1, Israel Barrantes 3 and Ulf Karsten 1 1 Department of Applied Ecology and Phycology, Institute of Biological Sciences, University of Rostock, 18059 Rostock, Germany; [email protected] (V.S.); [email protected] (U.K.) 2 Department of Biology, Botanical Institute, University of Cologne, 50674 Cologne, Germany 3 Research Group Translational Bioinformatics, Institute for Biostatistics and Informatics in Medicine and Ageing Research, Rostock University Medical Center, 18057 Rostock, Germany; [email protected] * Correspondence: [email protected] Abstract: Potash tailing piles located in Germany represent extremely hypersaline locations that negatively affect neighbouring environments and limit the development of higher vegetation. How- ever, biocrusts, as cryptogamic covers, inhabit some of these areas and provide essential ecological functions, but, nevertheless, they remain poorly described. Here, we applied high-throughput sequencing (HTS) and targeted four groups of microorganisms: bacteria, cyanobacteria, fungi and other eukaryotes. The sequencing of the 16S rRNA gene revealed the dominance of Proteobacteria, Cyanobacteria and Actinobacteria. Additionally, we applied yanobacteria-specific primers for a detailed assessment of the cyanobacterial community, which was dominated by members of the filamentous orders Synechococcales and Oscillatoriales. Furthermore, the majority of reads in the studied biocrusts obtained by sequencing of the 18S rRNA gene belonged to eukaryotic microalgae. In addition, sequencing of the internal rDNA transcribed spacer region (ITS) showed the dominance Citation: Pushkareva, E.; Sommer, V.; of Ascomycota within the fungal community. Overall, these molecular data provided the first detailed Barrantes, I.; Karsten, U. -
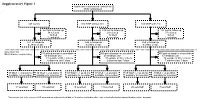
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Why the –Omic Future of Apicomplexa Should Include Gregarines Julie Boisard, Isabelle Florent
Why the –omic future of Apicomplexa should include Gregarines Julie Boisard, Isabelle Florent To cite this version: Julie Boisard, Isabelle Florent. Why the –omic future of Apicomplexa should include Gregarines. Biology of the Cell, Wiley, 2020, 10.1111/boc.202000006. hal-02553206 HAL Id: hal-02553206 https://hal.archives-ouvertes.fr/hal-02553206 Submitted on 24 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Article title: Why the –omic future of Apicomplexa should include Gregarines. Names of authors: Julie BOISARD1,2 and Isabelle FLORENT1 Authors affiliations: 1. Molécules de Communication et Adaptation des Microorganismes (MCAM, UMR 7245), Département Adaptations du Vivant (AVIV), Muséum National d’Histoire Naturelle, CNRS, CP52, 57 rue Cuvier 75231 Paris Cedex 05, France. 2. Structure et instabilité des génomes (STRING UMR 7196 CNRS / INSERM U1154), Département Adaptations du vivant (AVIV), Muséum National d'Histoire Naturelle, CP 26, 57 rue Cuvier 75231 Paris Cedex 05, France. Short Title: Gregarines –omics for Apicomplexa studies -

Protistology Molecular Phylogeny of Aphelidium Arduennense Sp. Nov
Protistology 13 (4), 192–198 (2019) Protistology Molecular phylogeny of Aphelidium arduennense sp. nov. – new representative of Aphelida (Opis- thosporidia) Victoria S. Tcvetkova1, Natalia A. Zorina1, Maria A. Mamkaeva1 and Sergey A. Karpov1,2 1 St. Petersburg State University, St. Petersburg 199034, Russia 2 Zoological Institute, Russian Academy of Sciences, St. Petersburg 199034, Russia | Submitted November 14, 2019 | Accepted December 2, 2019 | Summary Aphelids (Aphelida) are poorly known parasitoids of algae that have raised considerable interest because of their phylogenetic position as phagotrophic protists sister to Fungi. Together with Rozellida and Microsporidia they have been classified in the Opisthosporidia but seem to be more closely related to the Fungi rather than to the Cryptomycota and Microsporidia, the other members of the Opisthosporidia. Molecular environmental studies have revealed high genetic diversity within the aphelids, but only four genera have been described: Aphelidium, Amoeboaphelidium, Paraphelidium and Pseudaphelidium. Here, we describe the life cycle of a new species of Aphelidium, Aph. arduennense. Molecular phylogenetic analysis of its 18S rRNA indicates that Aph. arduennense is sister to Aph. tribonematis, and together with Aph. melosirae they form a monophyletic cluster. Within the aphelids, this cluster is distantly related to Paraphelidium and Amoeboaphelidium. Key words: aphelids, Holomycota, Opisthosporidia, Rozellosporidia, taxonomy Introduction Rozellosporidia (Cryptomycota) formed the super- phylum Opisthosporidia, the deepest branch Aphelids are a divergent group of intracellular of the Holomycota lineage, separated from the parasitoids of green, yellow-green and diatom algae Fungi (Karpov et al., 2014a; Letcher et al., 2015; (Gromov, 2000; Karpov et al., 2014a). The four 2017; Torruella et al., 2015). Several biological known genera have different ecological preferences: peculiarities of the aphelids do not conform the Aphelidium, Amoeboaphelidium and Paraphelidium classical definition of the Fungi. -

The Life Cycles of Cryptogams 7
Acta Botanica Malacitana, 16(1): 5-18 Málaga, 1991 E IE CYCES O CYOGAMS Peter R. BELL SUMMARY: Meiosis and karyogamy are recognized as control points in the life cycle of cryptogams. The control of meiosis is evidently complex and in yeast, and by analogy in all cryptogams, involves progressive gene activation. The causes of the delay in meiosis in diplohaplontic and diplontic organisms, and the manner in which the block is removed remain to be discovered. There is accumulating evidence that cytoplasmic RNA plays an important role in meiotic division. Many features of tn are still obscure. The tendency to oogamy has provided the opportunity for the laying down of long-lived messenger RNA in the abundant cytoplasm of the female gamete. The sporophytic nature of the developing zygote can in this way be partially pre-determined. There is evidence that this is the situation in the ferns. Specific molecules (probably arabino-galacto-proteins) on the surface of the plasma membrane are likely to account both for gametic selection, and the readiness with which appropriate gametes fuse. The dikaryotic condition indicates that nuclear fusion is not inevitable following plasmogamy. The ultimate fusion of the nuclei may result from quite simple changes in the nuclear surface. Exposure of lipid, for example, would lead to fusion as a result of hydrophobic forces. Aberrations of cryptogamic life cycles are numerous. The nuclear relationships of many aberrant cycles are unknown. In general it appears that the maintenance of sporophytic growth depends upon the presence of at least two sets of chromosomes. Conversely the maintenance of gametophytic growth in cultures obtained aposporously appears to be impossible in the presence of four sets of chromosomes, or more. -

S41467-021-25308-W.Pdf
ARTICLE https://doi.org/10.1038/s41467-021-25308-w OPEN Phylogenomics of a new fungal phylum reveals multiple waves of reductive evolution across Holomycota ✉ ✉ Luis Javier Galindo 1 , Purificación López-García 1, Guifré Torruella1, Sergey Karpov2,3 & David Moreira 1 Compared to multicellular fungi and unicellular yeasts, unicellular fungi with free-living fla- gellated stages (zoospores) remain poorly known and their phylogenetic position is often 1234567890():,; unresolved. Recently, rRNA gene phylogenetic analyses of two atypical parasitic fungi with amoeboid zoospores and long kinetosomes, the sanchytrids Amoeboradix gromovi and San- chytrium tribonematis, showed that they formed a monophyletic group without close affinity with known fungal clades. Here, we sequence single-cell genomes for both species to assess their phylogenetic position and evolution. Phylogenomic analyses using different protein datasets and a comprehensive taxon sampling result in an almost fully-resolved fungal tree, with Chytridiomycota as sister to all other fungi, and sanchytrids forming a well-supported, fast-evolving clade sister to Blastocladiomycota. Comparative genomic analyses across fungi and their allies (Holomycota) reveal an atypically reduced metabolic repertoire for sanchy- trids. We infer three main independent flagellum losses from the distribution of over 60 flagellum-specific proteins across Holomycota. Based on sanchytrids’ phylogenetic position and unique traits, we propose the designation of a novel phylum, Sanchytriomycota. In addition, our results indicate that most of the hyphal morphogenesis gene repertoire of multicellular fungi had already evolved in early holomycotan lineages. 1 Ecologie Systématique Evolution, CNRS, Université Paris-Saclay, AgroParisTech, Orsay, France. 2 Zoological Institute, Russian Academy of Sciences, St. ✉ Petersburg, Russia. 3 St. -

Assessing the Efficiency of Molecular Markers for the Species Identification of Gregarines Isolated from the Mealworm and Super Worm Midgut
Microorganisms 2018, 6, 119 1 of 20 Article Assessing the Efficiency of Molecular Markers for the Species Identification of Gregarines Isolated from the Mealworm and Super Worm Midgut Chiara Nocciolini 1, Claudio Cucini 2, Chiara Leo 2, Valeria Francardi 3, Elena Dreassi 4 and Antonio Carapelli 2,* 1 Polo d’Innovazione di Genomica Genetica e Biologia, 53100 Siena, Italy; [email protected] 2 Department of Life Sciences, University of Siena, 53100 Siena, Italy; [email protected] (C.C.); [email protected] (C.L.) 3 Consiglio per la ricerca in agricoltura e analisi dell’economia agraria – Centro di Difesa e Certificazione CREA-DC) Research Centre for Plant Protection and Certification, via di Lanciola 12/A, 50125 Firenze, Italy; [email protected] 4 Department of Biotechnology, Chemistry and Pharmacy, University of Siena, 53100 Siena, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0577-234-410 Received: 28 September 2018; Accepted: 23 November 2018; Published: 27 November 2018 Abstract: Protozoa, of the taxon Gregarinasina, are a heterogeneous group of Apicomplexa that includes ~1600 species. They are parasites of a large variety of both marine and terrestrial invertebrates, mainly annelids, arthropods and mollusks. Unlike coccidians and heamosporidians, gregarines have not proven to have a negative effect on human welfare; thus, they have been poorly investigated. This study focuses on the molecular identification and phylogeny of the gregarine species found in the midgut of two insect species that are considered as an alternative source of animal proteins for the human diet: the mealworm Tenebrio molitor, and the super-worm Zophobas atratus (Coleoptera: Tenebrionidae). -

Gregarines (Apicomplexa: Gregarinasina) Are Monoxenous Parasites of Invertebrates
Gregarines (Apicomplexa: Gregarinasina) are monoxenous parasites of invertebrates. Those found in sand flies (Diptera: Psychodidae) and mosquitoes (Diptera: Culicidae) used to be considered a single eugregarine genus Ascogregarina. Our phylogenetic analyses of the gregarine SSU rDNA, including newly obtained sequences of three species from sand flies, showed that mosquito and sand fly gregarines are closely related to neogregarines, and most importantly, they form two disparate monophyletic groups. Based on these molecular features, accompanied by biological differences, we established a new genus Psychodiella for the gregarines from sand flies, reserving the genus Ascogregarina for the mosquito gregarines. In the new genus, two new species Psychodiella sergenti from Phlebotomus sergenti and Psychodiella tobbi from Phlebotomus tobbi were described. They differ in the life cycles (sexual development of Ps. sergenti is triggered by a blood meal intake) and morphology of their life stages, mainly oocysts. The susceptibility of five sand fly species to both gregarines showed their strict host specificity, as they were able to fully develop and complete the life cycle only in their natural hosts. The life cycle of Ps. sergenti was studied in detail using various microscopical methods. Oocysts are attached to the chorion of sand fly eggs. Sporozoites, with a three- layered pellicle and mucron, attach to the 1st instar larval intestine but are never located intracellularly. In the 4th instar larvae, the gregarines occur in the ectoperitrophic space and later in the intestinal lumen. In adults, the parasites appear in the body cavity, and the sexual development of Ps. sergenti takes place only in blood-fed females; gametocysts attach to the accessory glands and oocysts are injected into their lumen. -

Interaction Between Metarhizium Anisopliae and Its Host, the Subterranean Termite Coptotermes Curvignathus During the Infection Process
biology Article Interaction between Metarhizium anisopliae and Its Host, the Subterranean Termite Coptotermes curvignathus during the Infection Process Samsuddin Ahmad Syazwan 1,2 , Shiou Yih Lee 1, Ahmad Said Sajap 1, Wei Hong Lau 3, Dzolkhifli Omar 3 and Rozi Mohamed 1,* 1 Department of Forest Science and Biodiversity, Faculty of Forestry and Environment, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] (S.A.S.); [email protected] (S.Y.L.); [email protected] (A.S.S.) 2 Mycology and Pathology Branch, Forest Biodiversity Division, Forest Research Institute Malaysia (FRIM), Kepong 52109, Malaysia 3 Department of Plant Protection, Faculty of Agriculture, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] (W.H.L.); zolkifl[email protected] (D.O.) * Correspondence: [email protected]; Tel.: +60-397-697-183 Simple Summary: The use of Metarhizium anisopliae as a biological control of insect pests has been experimented in the laboratory as well as in field trials. This includes against the termite Coptotermes curvignathus, however the results have varying degrees of success. One reason could be due to the lack of detailed knowledge on the molecular pathogenesis of M. anisopliae. In the current study, the conidial suspension of M. anisopliae isolate PR1 was first inoculated on the C. curvignathus, after which the pathogenesis was examined using two different approaches: electron microscopy and protein expression. At the initiation stage, the progression observed and documented including Citation: Syazwan, S.A.; Lee, S.Y.; adhesion, germination, and penetration of the fungus on the cuticle within 24 h after inoculation. Sajap, A.S.; Lau, W.H.; Omar, D.; Later, this was followed by colonization and spreading of the fungus at the cellular level.