Apicomplexa: Eugregarinida: Hirmocystidae) and Leidyana Haasi N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of Encyclopedia Boyong Sleman Insekta River As Alternative Learning Resources
PROC. INTERNAT. CONF. SCI. ENGIN. ISSN 2597-5250 Volume 3, April 2020 | Pages: 629-634 E-ISSN 2598-232X Development of Encyclopedia Boyong Sleman Insekta River as Alternative Learning Resources Rini Dita Fitriani*, Sulistiyawati Biological Education Faculty of Science and Technology, UIN Sunan Kalijaga Jl. Marsda Adisucipto Yogyakarta, Indonesia Email*: [email protected] Abstract. This study aims to determine the types of insects Coleoptera, Hemiptera, Odonata, Orthoptera and Lepidoptera in the Boyong River, Sleman Regency, Yogyakarta, to develop the Encyclopedia of the Boyong River Insect and to determine the quality of the encyclopedia developed. The method used in the research inventory of the types of insects Coleoptera, Hemiptera, Odonata, Orthoptera and Lepidoptera insects in the Boyong River survey method with the results of the study found 46 species of insects consisting of 2 Coleoptera Orders, 2 Hemiptera Orders, 18 orders of Lepidoptera in Boyong River survey method with the results of the research found 46 species of insects consisting of 2 Coleoptera Orders, 2 Hemiptera Orders, 18 orders of Lepidoptera in Boyong River survey method. odonata, 4 Orthopterous Orders and 20 Lepidopterous Orders from 15 families. The encyclopedia that was developed was created using the Adobe Indesig application which was developed in printed form. Testing the quality of the encyclopedia uses a checklist questionnaire and the results of the percentage of ideals from material experts are 91.1% with very good categories, 91.7% of media experts with very good categories, peer reviewers 92.27% with very good categories, biology teachers 88, 53% with a very good category and students 89.8% with a very good category. -

THE FLOUR BEETLES of the GENUS TRIBOLIUM by NEWELL E
.-;. , I~ 1I111~1a!;! ,I~, ,MICROCOPY RESOLUTION TEST ;CHART MICROCOPY RESOLUTION TEST CHART "NATlqNALBl!REAU PF.STANDARDS-1963-A 'NATIDNALBUREAU .OF STANDARD.S-.l963-A ':s, .' ...... ~~,~~ Technical BuIIetilL No. 498 '~: March: 1936 UNITED STATES DEPARThIENT OF AGRICULTURE WASHINGTON, D. C; THE FLOUR BEETLES OF THE GENUS TRIBOLIUM By NEWELL E. GOOD AssiStant ent()mo{:ofli~t, DiV;-8;.on of Cereal (lnd' Forage 11I_~eot In,l7estigatlo1ts; Bureau: 01 EntomolO!l1f and- Plu.nt Qua.ra-11ti;ne 1 CONTENTS Page lntrodu<:tfol1--___________ 1 .Li.f-e Jjjstory of TribfJli:/I.lIIi c(l-,tanellm SYll()nsm.!es and teennrcu.! descrtptkln;t !lnd T. c'mfu<.tlln'-_____________ 2.1 The egg______________________ 23 ofcles' the of Tribolitlmeconomjeal1y___________ important ~pe-_ The- lu.rviL____________-'__ 25 The- jl:enllB T,:ibolill.ln :lfucLclIY___ Thepnpa____________________ !~4 Key to; the .apede..; of TY~1Jfllif"'''-_ The ailult__________________ 36 Synonymies and: descrlpfions___ Interrelation witll. ot.he~ ullimals___ 44 History and economIc impormncll' ·of ).!edicuJ I:npona1H!e__________• 44 the genu!! TrilloLium________ 12 :Enem!"s. of Tri.ool'i:u:m. ~t:!"",-___ 44. Common: nll:mes________ 12 7'ri1Jolitlnt as a predil.tor____46 PlllceDfstrrhutlOll' pf !,rlgino ________________ of the genu$____-__ 1,1,13 ControlmetlllDrP$_____________ 47 Control in .flOur milL~__________ 47 Historical notes'-..._______ J .. Contrut of donr beetles fn hou!!<',;_ 49 l!D.teriala Wel!ted_________ 20 Summaxy__________________ .49 :Llterat'lre ctted__-_____________ 51 INTRODUCTION Flour and other prepared products frequently become inlested with sma.ll. reddish-brown beetles known as flour beetles. The...o:e beetles~ although very similar in size and a.ppearance, belong to the different though related genera T?ibolium. -

An Annotated Bibliography of Archaeoentomology
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Distance Master of Science in Entomology Projects Entomology, Department of 4-2020 An Annotated Bibliography of Archaeoentomology Diana Gallagher Follow this and additional works at: https://digitalcommons.unl.edu/entodistmasters Part of the Entomology Commons This Thesis is brought to you for free and open access by the Entomology, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Distance Master of Science in Entomology Projects by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Diana Gallagher Master’s Project for the M.S. in Entomology An Annotated Bibliography of Archaeoentomology April 2020 Introduction For my Master’s Degree Project, I have undertaken to compile an annotated bibliography of a selection of the current literature on archaeoentomology. While not exhaustive by any means, it is designed to cover the main topics of interest to entomologists and archaeologists working in this odd, dark corner at the intersection of these two disciplines. I have found many obscure works but some publications are not available without a trip to the Royal Society’s library in London or the expenditure of far more funds than I can justify. Still, the goal is to provide in one place, a list, as comprehensive as possible, of the scholarly literature available to a researcher in this area. The main categories are broad but cover the most important subareas of the discipline. Full books are far out-numbered by book chapters and journal articles, although Harry Kenward, well represented here, will be publishing a book in June of 2020 on archaeoentomology. -

Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Why the –Omic Future of Apicomplexa Should Include Gregarines Julie Boisard, Isabelle Florent
Why the –omic future of Apicomplexa should include Gregarines Julie Boisard, Isabelle Florent To cite this version: Julie Boisard, Isabelle Florent. Why the –omic future of Apicomplexa should include Gregarines. Biology of the Cell, Wiley, 2020, 10.1111/boc.202000006. hal-02553206 HAL Id: hal-02553206 https://hal.archives-ouvertes.fr/hal-02553206 Submitted on 24 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Article title: Why the –omic future of Apicomplexa should include Gregarines. Names of authors: Julie BOISARD1,2 and Isabelle FLORENT1 Authors affiliations: 1. Molécules de Communication et Adaptation des Microorganismes (MCAM, UMR 7245), Département Adaptations du Vivant (AVIV), Muséum National d’Histoire Naturelle, CNRS, CP52, 57 rue Cuvier 75231 Paris Cedex 05, France. 2. Structure et instabilité des génomes (STRING UMR 7196 CNRS / INSERM U1154), Département Adaptations du vivant (AVIV), Muséum National d'Histoire Naturelle, CP 26, 57 rue Cuvier 75231 Paris Cedex 05, France. Short Title: Gregarines –omics for Apicomplexa studies -
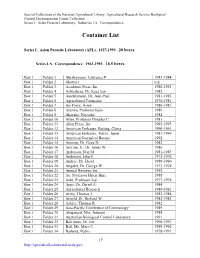
Container List
Special Collections of the National Agricultural Library: Agricultural Research Service Biological Control Documentation Center Collection Series I. Asian Parasite Laboratory. Subseries I.A. Correspondence. Container List Series I. Asian Parasite Laboratory (APL). 1927-1993. 20 boxes. Series I.A. Correspondence. 1963-1993. 16.5 boxes. Box 1 Folder 1 Abrahamson, Lawrence P. 1983-1984 Box 1 Folder 2 Abstract n.d. Box 1 Folder 3 Academic Press, Inc. 1986-1993 Box 1 Folder 4 Achterberg, Dr. Kees van 1982 Box 1 Folder 5 Aeschlimann, Dr. Jean-Paul 1981-1985 Box 1 Folder 6 Agricultural Counselor 1976-1981 Box 1 Folder 7 Air Force, Army 1980-1981 Box 1 Folder 8 Aizawa, Professor Keio 1986 Box 1 Folder 9 Akasaka, Naoyuki 1982 Box 1 Folder 10 Allen, Professor Douglas C. 1981 Box 1 Folder 11 Allen Press, Inc. 1992-1993 Box 1 Folder 12 American Embassy, Beijing, China 1990-1991 Box 1 Folder 13 American Embassy, Tokyo, Japan 1981-1990 Box 1 Folder 14 American Journal of Botany 1992 Box 1 Folder 15 Amman, Dr. Gene D. 1982 Box 1 Folder 16 Amrine, Jr., Dr. James W. 1986 Box 1 Folder 17 Anderson, Don M. 1981-1983 Box 1 Folder 18 Anderson, John F. 1975-1976 Box 1 Folder 19 Andres, Dr. Lloyd 1989-1990 Box 1 Folder 20 Angalet, Dr. George W. 1973-1978 Box 1 Folder 21 Annual Reviews Inc. 1992 Box 1 Folder 22 Ao, Professor Hsien-Bine 1988 Box 1 Folder 23 Aoki, Professor Joji 1977-1978 Box 1 Folder 24 Apps, Dr. Darrel A. 1984 Box 1 Folder 25 Agricultural Research 1980-1981 Box 1 Folder 26 Army, Thomas J. -

Tracing the Origin of Planktonic Protists in an Ancient Lake
microorganisms Article Tracing the Origin of Planktonic Protists in an Ancient Lake Nataliia V. Annenkova 1,* , Caterina R. Giner 2,3 and Ramiro Logares 2,* 1 Limnological Institute Siberian Branch of the Russian Academy of Sciences 3, Ulan-Batorskaya St., 664033 Irkutsk, Russia 2 Institute of Marine Sciences (ICM), CSIC, Passeig Marítim de la Barceloneta, 37-49, ES08003 Barcelona, Spain; [email protected] 3 Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver, BC V6T 1Z4, Canada * Correspondence: [email protected] (N.V.A.); [email protected] (R.L.) Received: 26 February 2020; Accepted: 7 April 2020; Published: 9 April 2020 Abstract: Ancient lakes are among the most interesting models for evolution studies because their biodiversity is the result of a complex combination of migration and speciation. Here, we investigate the origin of single celled planktonic eukaryotes from the oldest lake in the world—Lake Baikal (Russia). By using 18S rDNA metabarcoding, we recovered 1414 Operational Taxonomic Units (OTUs) belonging to protists populating surface waters (1–50 m) and representing pico/nano-sized cells. The recovered communities resembled other lacustrine freshwater assemblages found elsewhere, especially the taxonomically unclassified protists. However, our results suggest that a fraction of Baikal protists could belong to glacial relicts and have close relationships with marine/brackish species. Moreover, our results suggest that rapid radiation may have occurred among some protist taxa, partially mirroring what was already shown for multicellular organisms in Lake Baikal. We found 16% of the OTUs belonging to potential species flocks in Stramenopiles, Alveolata, Opisthokonta, Archaeplastida, Rhizaria, and Hacrobia. -

Herpetofauna and Aquatic Macro-Invertebrate Use of the Kino Environmental Restoration Project (KERP)
Herpetofauna and Aquatic Macro-invertebrate Use of the Kino Environmental Restoration Project (KERP) Tucson, Pima County, Arizona Prepared for Pima County Regional Flood Control District Prepared by EPG, Inc. JANUARY 2007 - Plma County Regional FLOOD CONTROL DISTRICT MEMORANDUM Water Resources Regional Flood Control District DATE: January 5,2007 TO: Distribution FROM: Julia Fonseca SUBJECT: Kino Ecosystem Restoration Project Report The Ed Pastor Environmental Restoration ProjectiKino Ecosystem Restoration Project (KERP) is becoming an extraordinary urban wildlife resource. As such, the Pima County Regional Flood Control District (PCRFCD) contracted with the Environmental Planning Group (EPG) to gather observations of reptiles, amphibians, and aquatic insects at KERP. Water quality was also examined. The purpose of the work was to provide baseline data on current wildlife use of the KERP site, and to assess water quality for post-project aquatic wildlife conditions. I additionally requested sampling of macroinvertebrates at Agua Caliente Park and Sweetwater Wetlands in hopes that the differences in aquatic wildlife among the three sites might provide insights into the different habitats offered by KERF'. The results One of the most important wildlife benefits that KERP provides is aquatic habitat without predatory bullfrogs and non- native fish. Most other constructed ponds and wetlands in Tucson, such as the Sweetwater Wetlands and Agua Caliente pond, are fuIl of non-native predators which devastate native fish, amphibians and aquatic reptiles. The KERP Wetlands may provide an opportunity for reestablishing declining native herpetofauna. Provided that non- native fish, bullfrogs or crayfish are not introduced, KERP appears to provide adequate habitat for Sonoran Mud Turtles (Kinosternon sonoriense), Lowland Leopard Frogs (Rana yavapaiensis), and Mexican Gartersnakes (Tharnnophis eques) and Southwestern Woodhouse Toad (Bufo woodhousii australis). -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Sarah A. Maxfield-Taylor for the degree of Master of Science in Entomology presented on March 26, 2014. Title: Natural Enemies of Native Bumble Bees (Hymenoptera: Apidae) in Western Oregon Abstract approved: _____________________________________________ Sujaya U. Rao Bumble bees (Hymenoptera: Apidae) are important native pollinators in wild and agricultural systems, and are one of the few groups of native bees commercially bred for use in the pollination of a range of crops. In recent years, declines in bumble bees have been reported globally. One factor implicated in these declines, believed to affect bumble bee colonies in the wild and during rearing, is natural enemies. A diversity of fungi, protozoa, nematodes, and parasitoids has been reported to affect bumble bees, to varying extents, in different parts of the world. In contrast to reports of decline elsewhere, bumble bees have been thriving in Oregon on the West Coast of the U.S.A.. In particular, the agriculturally rich Willamette Valley in the western part of the state appears to be fostering several species. Little is known, however, about the natural enemies of bumble bees in this region. The objectives of this thesis were to: (1) identify pathogens and parasites in (a) bumble bees from the wild, and (b) bumble bees reared in captivity and (2) examine the effects of disease on bee hosts. Bumble bee queens and workers were collected from diverse locations in the Willamette Valley, in spring and summer. Bombus mixtus, Bombus nevadensis, and Bombus vosnesenskii collected from the wild were dissected and examined for pathogens and parasites, and these organisms were identified using morphological and molecular characteristics. -
STORGARD Insect Identification Poster
® IPM PARTNER® INSECT IDENTIFICATION GUIDE ® Name Photo Size Color Typical Favorite Attracted Geographic Penetrate Product Recommendation (mm) Life Cycle Food to Light Distribution Packages MOTHS Almond Moth 14-20 Gray 25-30 Dried fruit Yes General Yes, Cadra cautella days and grain larvae only STORGARD® II STORGARD® III CIDETRAK® IMM Also available in QUICK-CHANGE™ Also available in QUICK-CHANGE™ (Mating Disruptant) Angoumois 28-35 Yes, Grain Moth 13-17 Buff days Whole grain Yes General larvae only Sitotroga cerealella STORGARD® II STORGARD® III Casemaking 30-60 Wool, natural Yes, Clothes Moth 11 Brownish days fibers and hair Yes General larvae only Tinea pellionella STORGARD® II STORGARD® III European Grain Moth 13-17 White & 90-300 Grain Yes Northern Yes, Nemapogon granellus brown days larvae only STORGARD® II STORGARD® III Copper Indianmeal Moth Broken or 8-10 red & silver 28-35 processed Yes General Yes, Plodia interpunctella days larvae only gray grain STORGARD® II STORGARD® III CIDETRAK® IMM Also available in QUICK-CHANGE™ Also available in QUICK-CHANGE™ (Mating Disruptant) Mediterranean Gray & Flour and Flour Moth 10-15 30-180 processed Yes General Yes, black days larvae only Ephestia kuehniella cereal grain STORGARD® II STORGARD® III CIDETRAK® IMM Also available in QUICK-CHANGE™ Also available in QUICK-CHANGE™ (Mating Disruptant) Raisin Moth Drying and 12-20 Gray 32 days Yes General Yes, dried fruit larvae only Cadra figulilella STORGARD® II STORGARD® III CIDETRAK® IMM Also available in QUICK-CHANGE™ Also available in QUICK-CHANGE™ -

Catalogue of Protozoan Parasites Recorded in Australia Peter J. O
1 CATALOGUE OF PROTOZOAN PARASITES RECORDED IN AUSTRALIA PETER J. O’DONOGHUE & ROBERT D. ADLARD O’Donoghue, P.J. & Adlard, R.D. 2000 02 29: Catalogue of protozoan parasites recorded in Australia. Memoirs of the Queensland Museum 45(1):1-164. Brisbane. ISSN 0079-8835. Published reports of protozoan species from Australian animals have been compiled into a host- parasite checklist, a parasite-host checklist and a cross-referenced bibliography. Protozoa listed include parasites, commensals and symbionts but free-living species have been excluded. Over 590 protozoan species are listed including amoebae, flagellates, ciliates and ‘sporozoa’ (the latter comprising apicomplexans, microsporans, myxozoans, haplosporidians and paramyxeans). Organisms are recorded in association with some 520 hosts including mammals, marsupials, birds, reptiles, amphibians, fish and invertebrates. Information has been abstracted from over 1,270 scientific publications predating 1999 and all records include taxonomic authorities, synonyms, common names, sites of infection within hosts and geographic locations. Protozoa, parasite checklist, host checklist, bibliography, Australia. Peter J. O’Donoghue, Department of Microbiology and Parasitology, The University of Queensland, St Lucia 4072, Australia; Robert D. Adlard, Protozoa Section, Queensland Museum, PO Box 3300, South Brisbane 4101, Australia; 31 January 2000. CONTENTS the literature for reports relevant to contemporary studies. Such problems could be avoided if all previous HOST-PARASITE CHECKLIST 5 records were consolidated into a single database. Most Mammals 5 researchers currently avail themselves of various Reptiles 21 electronic database and abstracting services but none Amphibians 26 include literature published earlier than 1985 and not all Birds 34 journal titles are covered in their databases. Fish 44 Invertebrates 54 Several catalogues of parasites in Australian PARASITE-HOST CHECKLIST 63 hosts have previously been published. -

An Overview of Systematics Studies Concerning the Insect Fauna of British Columbia
J ENTOMOL. Soc. B RIT. COLU MBIA 9 8. DECDmER 200 1 33 An overview of systematics studies concerning the insect fauna of British Columbia ROBERT A. CANNINGS ROYAL BRITISI-I COLUMBIA MUSEUM, 675 BELLEVILLE STREET, VICTORIA, BC, CANADA V8W 9W2 GEOFFREY G.E. SCUDDER DEPARTMENT OF ZOOLOGY, UNIVERSITY OF BRITISI-I COLUMBIA, VANCOUVER, BC, CANADA V6T IZ4 INTRODUCTION This summary of insect systematics pertaining to British Co lumbia is not intended as an hi storical account of entomologists and their work, but rath er is an overview of th e more important studies and publications dealing with the taxonomy, identifi cati on, distribution and faunistics of BC species. Some statistics on th e known size of various taxa are also give n. Many of the systematic references to th e province's insects ca nnot be presented in such a short summary as thi s and , as a res ult, the treatment is hi ghl y se lec tive. It deals large ly with publications appearing after 1950. We examine mainly terrestrial groups. Alth ough Geoff Scudder, Professor of Zoo logy at the University of Briti sh Co lumbi a, at Westwick Lake in the Cariboo, May 1970. Sc udder is a driving force in man y facets of in sect systemat ics in Briti sh Co lum bia and Canada. He is a world authority on th e Hemiptera. Photo: Rob Ca nnin gs. 34 J ENTOMOL. Soc BR IT. COLUMBIA 98, DECEMBER200 1 we mention the aquatic orders (those in which the larv ae live in water but the adults are aerial), they are more fully treated in the companion paper on aquatic in sects in thi s issue (Needham et al.) as are the major aquatic families of otherwise terrestrial orders (e.g.