Hallucinogen-Like Actions of 5-Methoxy-N,N-Diisopropyltryptamine in Mice and Rats ⁎ W.E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

21 CFR Ch. II (4–1–10 Edition) § 1308.12
§ 1308.12 21 CFR Ch. II (4–1–10 Edition) Some trade and other names: N,N- whenever the existence of such salts, Diethyltryptamine; DET isomers, and salts of isomers is possible (18) Dimethyltryptamine ................................................. 7435 Some trade or other names: DMT within the specific chemical designa- (19) 5-methoxy-N,N-diisopropyltryptamine (other tion: name: 5-MeO-DIPT) ................................................... 7439 (1) gamma-hydroxybutyric acid (some other names in- (20) Ibogaine .................................................................. 7260 clude GHB; gamma-hydroxybutyrate; 4- Some trade and other names: 7-Ethyl- hydroxybutyrate; 4-hydroxybutanoic acid; sodium 6,6b,7,8,9,10,12,13-octahydro-2-methoxy-6,9- oxybate; sodium oxybutyrate) .................................... 2010 methano-5H-pyrido [1′, 2′:1,2] azepino [5,4-b] (2) Mecloqualone ........................................................... 2572 indole; Tabernanthe iboga (3) Methaqualone ........................................................... 2565 (21) Lysergic acid diethylamide ..................................... 7315 (22) Marihuana .............................................................. 7360 (f) Stimulants. Unless specifically ex- (23) Mescaline ............................................................... 7381 cepted or unless listed in another (24) Parahexyl—7374; some trade or other names: 3- schedule, any material, compound, Hexyl-1-hydroxy-7,8,9,10-tetrahydro-6,6,9-trimethyl- 6H-dibenzo[b,d]pyran; Synhexyl. mixture, -
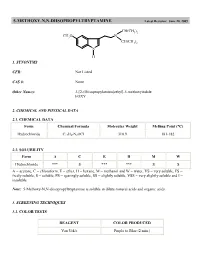
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo Jose A
Morales-Garcia et al. Translational Psychiatry (2020) 10:331 https://doi.org/10.1038/s41398-020-01011-0 Translational Psychiatry ARTICLE Open Access N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo Jose A. Morales-Garcia 1,2,3,4, Javier Calleja-Conde 5, Jose A. Lopez-Moreno 5, Sandra Alonso-Gil1,2, Marina Sanz-SanCristobal1,2, Jordi Riba6 and Ana Perez-Castillo 1,2,4 Abstract N,N-dimethyltryptamine (DMT) is a component of the ayahuasca brew traditionally used for ritual and therapeutic purposes across several South American countries. Here, we have examined, in vitro and vivo, the potential neurogenic effect of DMT. Our results demonstrate that DMT administration activates the main adult neurogenic niche, the subgranular zone of the dentate gyrus of the hippocampus, promoting newly generated neurons in the granular zone. Moreover, these mice performed better, compared to control non-treated animals, in memory tests, which suggest a functional relevance for the DMT-induced new production of neurons in the hippocampus. Interestingly, the neurogenic effect of DMT appears to involve signaling via sigma-1 receptor (S1R) activation since S1R antagonist blocked the neurogenic effect. Taken together, our results demonstrate that DMT treatment activates the subgranular neurogenic niche regulating the proliferation of neural stem cells, the migration of neuroblasts, and promoting the generation of new neurons in the hippocampus, therefore enhancing adult neurogenesis and -

Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans
molecules Review Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans Paul Cumming 1,2,* , Milan Scheidegger 3 , Dario Dornbierer 3, Mikael Palner 4,5,6 , Boris B. Quednow 3,7 and Chantal Martin-Soelch 8 1 Department of Nuclear Medicine, Bern University Hospital, CH-3010 Bern, Switzerland 2 School of Psychology and Counselling, Queensland University of Technology, Brisbane 4059, Australia 3 Department of Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital of the University of Zurich, CH-8032 Zurich, Switzerland; [email protected] (M.S.); [email protected] (D.D.); [email protected] (B.B.Q.) 4 Odense Department of Clinical Research, University of Southern Denmark, DK-5000 Odense, Denmark; [email protected] 5 Department of Nuclear Medicine, Odense University Hospital, DK-5000 Odense, Denmark 6 Neurobiology Research Unit, Copenhagen University Hospital, DK-2100 Copenhagen, Denmark 7 Neuroscience Center Zurich, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8058 Zurich, Switzerland 8 Department of Psychology, University of Fribourg, CH-1700 Fribourg, Switzerland; [email protected] * Correspondence: [email protected] or [email protected] Abstract: Hallucinogens are a loosely defined group of compounds including LSD, N,N- dimethyltryptamines, mescaline, psilocybin/psilocin, and 2,5-dimethoxy-4-methamphetamine (DOM), Citation: Cumming, P.; Scheidegger, which can evoke intense visual and emotional experiences. We are witnessing a renaissance of re- M.; Dornbierer, D.; Palner, M.; search interest in hallucinogens, driven by increasing awareness of their psychotherapeutic potential. Quednow, B.B.; Martin-Soelch, C. As such, we now present a narrative review of the literature on hallucinogen binding in vitro and Molecular and Functional Imaging ex vivo, and the various molecular imaging studies with positron emission tomography (PET) or Studies of Psychedelic Drug Action in single photon emission computer tomography (SPECT). -

NIDA Drug Supply Program Catalog, 25Th Edition
RESEARCH RESOURCES DRUG SUPPLY PROGRAM CATALOG 25TH EDITION MAY 2016 CHEMISTRY AND PHARMACEUTICS BRANCH DIVISION OF THERAPEUTICS AND MEDICAL CONSEQUENCES NATIONAL INSTITUTE ON DRUG ABUSE NATIONAL INSTITUTES OF HEALTH DEPARTMENT OF HEALTH AND HUMAN SERVICES 6001 EXECUTIVE BOULEVARD ROCKVILLE, MARYLAND 20852 160524 On the cover: CPK rendering of nalfurafine. TABLE OF CONTENTS A. Introduction ................................................................................................1 B. NIDA Drug Supply Program (DSP) Ordering Guidelines ..........................3 C. Drug Request Checklist .............................................................................8 D. Sample DEA Order Form 222 ....................................................................9 E. Supply & Analysis of Standard Solutions of Δ9-THC ..............................10 F. Alternate Sources for Peptides ...............................................................11 G. Instructions for Analytical Services .........................................................12 H. X-Ray Diffraction Analysis of Compounds .............................................13 I. Nicotine Research Cigarettes Drug Supply Program .............................16 J. Ordering Guidelines for Nicotine Research Cigarettes (NRCs)..............18 K. Ordering Guidelines for Marijuana and Marijuana Cigarettes ................21 L. Important Addresses, Telephone & Fax Numbers ..................................24 M. Available Drugs, Compounds, and Dosage Forms ..............................25 -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

Urinary Excretion Profiles of 5-Methoxy-N,N
Journal of Health Science, 53(5) 585–590 (2007) 585 Urinary Excretion Profiles of 60 hr for 6-OH-5-MeO-DIPT and 5-MeO-NIPT. 5-Methoxy-N,N- Key words —— 5-methoxy-N,N-diisopropyltryptamine, diisopropyltryptamine and Its excretion profile, enzymatic hydrolysis, urine analysis, Relevant Metabolites in Humans forensic science ∗ Tooru Kamata, Munehiro Katagi, INTRODUCTION Hiroe Kamata, Akihiro Miki, Noriaki Shima, Kei Zaitsu, Mayumi Nishikawa, 5-Methoxy-N,N-diisopropyltryptamine (5- and Hitoshi Tsuchihashi MeO-DIPT) (Fig. 1) is a psychotomimetic tryptamine derivative first synthesized and re- Forensic Science Laboratory, Osaka Prefectural Police Head- ported by Shulgin and Carter in 1980.1) It causes quarters, 1–3–18, Hommachi, Chuo-ku, Osaka 541–0053, mydriasis, and high dosage produces nausea, jaw Japan clenching, muscle tension, and overt hallucina- (Received March 5, 2007; Accepted June 14, 2007) tions with both auditory and visual distortions.2) 5-MeO-DIPT has been abused since the 1990s in 5-Methoxy-N,N-diisopropyltryptamine (5-MeO- many countries under the name “Foxy” or “Foxy DIPT), a psychotomimetic tryptamine derivative, Methoxy.” In 2005, this drug was banned in Japan and its relevant metabolites have been determined in under the Narcotics and Psychotropics Control Law eleven urine specimens from six 5-MeO-DIPT users, to prevent its rapid spread as a “recreational drug.” and their excretion profiles have been investigated For the control of drugs of abuse, careful inves- by gas chromatography/mass spectrometry (GC/MS) tigation on the metabolism and excretion of the drug and liquid chromatography/mass spectrometry and relevant metabolites in humans, as well as the (LC/MS). -

Alcohol and Drug Abuse Subchapter 9
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four-hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A. -

Recreational Use, Analysis and Toxicity of Tryptamines
Send Orders for Reprints to [email protected] 26 Current Neuropharmacology, 2015, 13, 26-46 Recreational Use, Analysis and Toxicity of Tryptamines Roberta Tittarelli1, Giulio Mannocchi1, Flaminia Pantano1 and Francesco Saverio Romolo1,2,* 1Legal Medicine Section, Department of Anatomical, Histological, Forensic Medicine and Orthopedic Sciences, “Sapienza” University of Rome, Viale Regina Elena, 336, 00161 Rome, Italy; 2Institut de Police Scientifique, Université de Lausanne, Batochime, 1015 Lausanne, Switzerland Abstract: The definition New psychoactive substances (NPS) refers to emerging drugs whose chemical structures are similar to other psychoactive compounds but not identical, representing a “legal” alternative to internationally controlled drugs. There are many categories of NPS, such as synthetic cannabinoids, synthetic cathinones, phenylethylamines, piperazines, ketamine derivatives and tryptamines. Tryptamines are naturally occurring compounds, which can derive from the amino acid tryptophan by several biosynthetic pathways: their structure is a combination of a benzene ring Roberta Tittarelli and a pyrrole ring, with the addition of a 2-carbon side chain. Tryptamines include serotonin and melatonin as well as other compounds known for their hallucinogenic properties, such as psilocybin in ‘Magic mushrooms’ and dimethyltryptamine (DMT) in Ayahuasca brews. Aim: To review the scientific literature regarding tryptamines and their derivatives, providing a summary of all the available information about the structure of these compounds, their effects in relationship with the routes of administration, their pharmacology and toxicity, including articles reporting cases of death related to intake of these substances. Methods: A comprehensive review of the published scientific literature was performed, using also non peer-reviewed information sources, such as books, government publications and drug user web fora. -

Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them, and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A.