21 CFR Ch. II (4–1–10 Edition) § 1308.12
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zoletil 100 Injectable Anaesthetic/Sedative for Dogs, Cats, Zoo and Wild Animals
Zoletil 100 Injectable Anaesthetic/Sedative for Dogs, Cats, Zoo and Wild Animals Virbac (Australia) Pty Limited Chemwatch Hazard Alert Code: 1 Chemwatch: 6978267 Issue Date: 11/01/2019 Version No: 4.1.16.10 Print Date: 08/31/2021 Safety Data Sheet according to WHS Regulations (Hazardous Chemicals) Amendment 2020 and ADG requirements L.GHS.AUS.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking Product Identifier Product name Zoletil 100 Injectable Anaesthetic/Sedative for Dogs, Cats, Zoo and Wild Animals Chemical Name Not Applicable Synonyms APVMA No.: 38837 Chemical formula Not Applicable Other means of identification Not Available Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses For the anaesthesia and immobilisation of dogs, cats, zoo and wild animals. Details of the supplier of the safety data sheet Registered company name Virbac (Australia) Pty Limited Address 361 Horsley Road Milperra NSW 2214 Australia Telephone 1800 242 100 Fax +61 2 9772 9773 Website au.virbac.com Email [email protected] Emergency telephone number Association / Organisation Poisons Information Centre Emergency telephone 13 11 26 numbers Other emergency telephone Not Available numbers SECTION 2 Hazards identification Classification of the substance or mixture NON-HAZARDOUS CHEMICAL. NON-DANGEROUS GOODS. According to the WHS Regulations and the ADG Code. ChemWatch Hazard Ratings Min Max Flammability 1 Toxicity 1 0 = Minimum Body Contact 1 1 = Low 2 = Moderate Reactivity 1 3 = High Chronic 0 4 = Extreme Poisons Schedule S4 Classification [1] Not Applicable Label elements Hazard pictogram(s) Not Applicable Signal word Not Applicable Hazard statement(s) Not Applicable Precautionary statement(s) Prevention Not Applicable Precautionary statement(s) Response Not Applicable Precautionary statement(s) Storage Page 1 continued.. -

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

Patient Risks & Preparation for a Successful Sedation Or Anesthetic Event
Patient risks & Sleep preparation for a successful well… sedation or anesthetic event Odette O, DVM, DACVAA ▪ Sedation versus general anesthetic: what are the considerations? ▪ What are the risks (literally) of sedation &/or anesthesia? ▪ Optimize patient preparation prior to sedation/anesthesia whenever possible! ▪ Be prepared: Who? What? Where? When? Why? ▪ Troubleshooting a rough recovery… ▪ ↑ risk of mortality seen with increasing ASA status ▪ Importance of patient evaluation and stabilization PRIOR to commencement of procedure ▪ Identify risk factors and monitor carefully ▪ Largest proportion of deaths in post-procedure period ▪ Continued patient monitoring & support vital • Diagnostic Imaging • Radiographs, CT, U/S • Biopsies • Small wound repair • Bandaging • Convenience • Faster recovery times • Reversible options • ↓ $ • ↑ margin of safety ▪ Reversible unconsciousness ▪ Amnesia ▪ Analgesia ▪ Muscle relaxation ▪ Perform a procedure ▪ w/o suffering ▪ Safety ▪ Patient ▪ Veterinary Care Provider(s) ▪ Multi-modal approach ▪ DO NOT “mask down” (canine/feline) patients! ▪ Patient & occupational safety concerns ▪ MAC (minimum alveolar concentration) = amount of inhalant needed for 50% of patients non- responsive to supramaximal stimulus ▪ Isoflurane: ≈ 1.3% canine, ≈1.6% feline ▪ Sevoflurane: ≈ 2.3% canine, ≈ 3% feline ▪ allows estimate of amount inhalant required ▪ factors: procedure, patient pre-med response, inhalant ▪ Minimal calculations needed ▪ Inhalant effective in every species we encounter ▪ Predictable effects on most patients, -

Download Product Insert (PDF)
PRODUCT INFORMATION Tiletamine (hydrochloride) Item No. 19794 CAS Registry No.: 14176-50-2 Formal Name: 2-(ethylamino)-2-(2- thienyl)-cyclohexanone, S monohydrochloride Synonyms: CI-634, CL-399, CN 54521-2, HN NSC 167740 O MF: C12H17NOS • HCl FW: 259.8 Purity: ≥98% • HCl UV/Vis.: λmax: 236 nm Supplied as: A crystalline solid Storage: -20°C Stability: As supplied, 2 years from the QC date provided on the Certificate of Analysis, when stored properly Description Tiletamine (hydrochloride) (Item No. 19794) is an analytical reference standard that is structurally classified as an arylcyclohexylamine. It is a non-competitive NMDA receptor antagonist used in veterinary medicine for its anesthetic and sedative properties, which are comparable to ketamine (Item No. 11630).1,2 Abuse of this compound has been documented.2 It is intended for forensic and research purposes only. References 1. Klockgether, T., Turski, L., Schwarz, M., et al. Paradoxical convulsant action of a novel non-competitive N-methyl-D-aspartate (NMDA) antagonist, tiletamine. Brain Res. 461(2), 343-348 (1988). 2. Quail, M. T., Weimersheimer, P., Woolf, A. D., et al. Abuse of telazol: An animal tranquilizer. J. Toxicol. Clin. Toxicol. 39(4), 399-402 (2001). WARNING CAYMAN CHEMICAL THIS PRODUCT IS FOR RESEARCH ONLY - NOT FOR HUMAN OR VETERINARY DIAGNOSTIC OR THERAPEUTIC USE. 1180 EAST ELLSWORTH RD SAFETY DATA ANN ARBOR, MI 48108 · USA This material should be considered hazardous until further information becomes available. Do not ingest, inhale, get in eyes, on skin, or on clothing. Wash thoroughly after handling. Before use, the user must review the complete Safety Data Sheet, which has been sent via email to your institution. -

The Role of the N-Methyl-D-Aspartate Receptors in Social Behavior in Rodents
International Journal of Molecular Sciences Review The Role of the N-Methyl-D-Aspartate Receptors in Social Behavior in Rodents Iulia Zoicas * and Johannes Kornhuber Department of Psychiatry and Psychotherapy, University Hospital, Friedrich-Alexander-University Erlangen-Nuremberg, 91054 Erlangen, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-9131-85-46005; Fax: +49-9131-85-36381 Received: 8 October 2019; Accepted: 5 November 2019; Published: 9 November 2019 Abstract: The appropriate display of social behaviors is essential for the well-being, reproductive success and survival of an individual. Deficits in social behavior are associated with impaired N-methyl-D-aspartate (NMDA) receptor-mediated neurotransmission. In this review, we describe recent studies using genetically modified mice and pharmacological approaches which link the impaired functioning of the NMDA receptors, especially of the receptor subunits GluN1, GluN2A and GluN2B, to abnormal social behavior. This abnormal social behavior is expressed as impaired social interaction and communication, deficits in social memory, deficits in sexual and maternal behavior, as well as abnormal or heightened aggression. We also describe the positive effects of pharmacological stimulation of the NMDA receptors on these social deficits. Indeed, pharmacological stimulation of the glycine-binding site either by direct stimulation or by elevating the synaptic glycine levels represents a promising strategy for the normalization of genetically-induced, pharmacologically-induced or innate deficits in social behavior. We emphasize on the importance of future studies investigating the role of subunit-selective NMDA receptor ligands on different types of social behavior to provide a better understanding of the underlying mechanisms, which might support the development of selective tools for the optimized treatment of disorders associated with social deficits. -

Anesthetic and Physiologic Effects of Tiletamine, Zolazepam, Ketamine, and Xylazine Combination (TKX) in Feral Cats Undergoing Surgical Sterilization
Journal of Feline Medicine and Surgery (2004) 6, 297e303 www.elsevier.com/locate/jfms Anesthetic and physiologic effects of tiletamine, zolazepam, ketamine, and xylazine combination (TKX) in feral cats undergoing surgical sterilization Alexis M. Cistolaa, Francis J. Golderb, Lisa A. Centonzea, ) Lindsay W. McKaya, Julie K. Levya, aDepartment of Small Animal Clinical Sciences, PO Box 100126, College of Veterinary Medicine, University of Florida, Gainesville, FL 32610, USA bDepartment of Comparative Bioscience, College of Veterinary Medicine, University of Wisconsin, Madison, WI 53706, USA Revised 23 November 2003; accepted 26 November 2003 Summary Tiletamine (12.5 mg), zolazepam (12.5 mg), ketamine (20 mg), and xylazine (5mg)(TKX;0.25ml,IM)combinationwasevaluatedasananestheticin22maleand67 female adult feral cats undergoing sterilization at high-volume sterilization clinics. Cats were not intubated and breathed room air. Oxygen saturation (SpO2), mean blood pres- sure (MBP), heart rate (HR), respiration rate (RR), and core body temperature were re- corded. Yohimbine (0.25 ml, 0.5 mg, IV) was administered at the completion of surgery. TKXproducedrapidonsetoflateralrecumbency(4G1 min) and surgical anesthesia of sufficient duration to complete surgical procedures in 92% of cats. SpO2 measured via a lingual pulse oximeter probe averaged 92G3% in male cats and 90G4% in females. SpO2 fell below 90% at least once in most cats. MBPmeasuredbyoscillometryaveraged 136G30 mm Hg in males and 113G29 mm Hg in females. MBP increased at the onset of surgical stimulation suggesting incomplete anti-nociceptive properties. HR averaged 156G19bpm,andRRaveraged18G8 bpm. Neither parameter varied between males and females or over time. Body temperature decreased significantly over time, declining to 38.0G0.8 (C at the time of reversal in males and 36.6G0.8 (Catthetimeofreversal in females. -

Hallucinogen-Like Actions of 5-Methoxy-N,N-Diisopropyltryptamine in Mice and Rats ⁎ W.E
Pharmacology, Biochemistry and Behavior 83 (2006) 122–129 www.elsevier.com/locate/pharmbiochembeh Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats ⁎ W.E. Fantegrossi a,b, , A.W. Harrington b, C.L. Kiessel b, J.R. Eckler c, R.A. Rabin c, J.C. Winter c, A. Coop d, K.C. Rice e, J.H. Woods b a Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, GA 30322, United States b Department of Pharmacology, University of Michigan Medical School, Ann Arbor, MI, United States c Department of Pharmacology and Toxicology, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY, United States d Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, MD, United States e Laboratory of Medicinal Chemistry, NIDDK, National Institutes of Health, Bethesda, MD Received 2 June 2005; received in revised form 7 December 2005; accepted 29 December 2005 Available online 3 February 2006 Abstract Few studies have examined the effects of 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT) in vivo. In these studies, 5-MeO-DIPT was tested in a drug-elicited head twitch assay in mice where it was compared to the structurally similar hallucinogen N,N-dimethyltryptamine (N,N- DMT) and challenged with the selective serotonin (5-HT)2A antagonist M100907, and in a lysergic acid diethylamide (LSD) discrimination assay in rats where its subjective effects were challenged with M100907 or the 5-HT1A selective antagonist WAY-100635. Finally, the affinity of 5- MeO-DIPT for three distinct 5-HT receptors was determined in rat brain. -
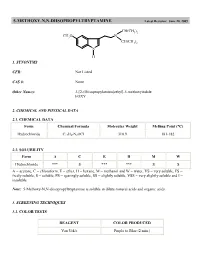
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Rachel Fowler 3Rd Floor Seacole Building 2
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Rachel Fowler 3rd Floor Seacole Building 2. Marsham Street London SW1P 4DF 020 7035 0454 Email: [email protected] Rt Hon. Theresa May MP Home Office 2 Marsham Street London SW1P 4DF 18th October 2012 Dear Home Secretary, In March 2012 the ACMD advised that methoxetamine be subject to a temporary class drug order. Methoxetamine was marketed as a legal alternative to ketamine until a temporary class drug order was implemented in April 2012. As is now required, the ACMD has followed its initial assessment with a consideration of methoxetamine in the context of the Misuse of Drugs Act 1971; I enclose the report with this letter. The chemical structure of methoxetamine bears a close resemblance to that of both ketamine and phencyclidine (PCP, „Angel Dust‟, a class A drug), which both produce well- documented and serious adverse effects following both acute and chronic usage. Users report that the effects of methoxetamine are similar to those of ketamine, however, some users report that the effects are of longer duration.The harmful effects reported include severe dissociation, cardiovascular symptoms, paranoid thoughts and unpleasant hallucinations. The first analytically confirmed series reported by Guy‟s and St Thomas‟ NHS Foundation Trust, London in 2011, was of three individuals who presented having self-reported use of methoxetamine. All three presented with a ketamine-like dissociative state, but also had significant stimulant effects with agitation and cardiovascular effects including tachycardia and hypertension. Toxicological screening of serum samples confirmed methoxetamine use in two of the cases. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo Jose A
Morales-Garcia et al. Translational Psychiatry (2020) 10:331 https://doi.org/10.1038/s41398-020-01011-0 Translational Psychiatry ARTICLE Open Access N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo Jose A. Morales-Garcia 1,2,3,4, Javier Calleja-Conde 5, Jose A. Lopez-Moreno 5, Sandra Alonso-Gil1,2, Marina Sanz-SanCristobal1,2, Jordi Riba6 and Ana Perez-Castillo 1,2,4 Abstract N,N-dimethyltryptamine (DMT) is a component of the ayahuasca brew traditionally used for ritual and therapeutic purposes across several South American countries. Here, we have examined, in vitro and vivo, the potential neurogenic effect of DMT. Our results demonstrate that DMT administration activates the main adult neurogenic niche, the subgranular zone of the dentate gyrus of the hippocampus, promoting newly generated neurons in the granular zone. Moreover, these mice performed better, compared to control non-treated animals, in memory tests, which suggest a functional relevance for the DMT-induced new production of neurons in the hippocampus. Interestingly, the neurogenic effect of DMT appears to involve signaling via sigma-1 receptor (S1R) activation since S1R antagonist blocked the neurogenic effect. Taken together, our results demonstrate that DMT treatment activates the subgranular neurogenic niche regulating the proliferation of neural stem cells, the migration of neuroblasts, and promoting the generation of new neurons in the hippocampus, therefore enhancing adult neurogenesis and -

Outline for Controlled Substances Program
Environmental Health and Safety Controlled Substances Program Date of Issuance: Review Date: 10/1/2019 (no changes) 10/01/2018 Revision Number: Initial Prepared by: EH&S Table of Contents HEADINGS Introduction Applicability Responsibilities Registration Requirements Authorized Use Ordering/Purchasing Administering and Dispensing Inventory Procedures (Continuing Records) Security Disposal FORMS: Registering or renewing a DEA or state license (CMU) Controlled Substances Authorized users list (CMU) Employee questionnaire for those with access to controlled substances (CMU) Record of Form 222 use (Order form) (CMU) Records of Controlled Substance Purchases (CMU) Record of Controlled Substance Administering and dispensing (CMU) Controlled Substance Physical Inventory (CMU) DEA Registration of Persons doing research or analysis (Form 225) DEA Registration of Dispensers (Form 224) DEA Registration Instructional (Form 224 and 226 to renew) DEA Report of loss or theft (Form 106) DEA Report of drugs surrendered (From 41) DEA SCHEDULES: Schedule I Schedule II Schedule III Schedule IV Schedule V INTRODUCTION State and Federal regulations have been promulgated concerning the use and handling of US Department of Justice Drug Enforcement Administration (DEA) controlled substances. These regulations are in place to address materials which are or have the potential to be addictive or habit forming. These substances have been categorized into “schedules” that have been created by the DEA to reflect their level of concern. The “Carnegie Mellon University DEA Controlled Substances Program” is intended to ensure that Carnegie Mellon University is in compliance with our regulatory requirements. Required activities under the DEA include: 1. Registration of your work with the DEA and with Carnegie Mellon’s Department of Environmental Health and Safety (EH&S).