House Bill No. 1176
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011)
EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011) 912 final COMMISSION STAFF WORKING PAPER on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances Accompanying the document REPORT FROM THE COMMISSION on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances {COM(2011) 430 final} EN EN TABLE OF CONTENTS 1. Introduction...................................................................................................................3 2. Methodology.................................................................................................................4 3. Key findings from the 2002 evaluation of the Joint Action on synthetic drugs ...........5 4. Overview of notifications, types of substances and trends at EU level 2005-2010......7 5. Other EU legislation relevant for the regulation of new psychoactive substances.....12 6. Functioning of the Council Decision on new psychoactive substances .....................16 7. Findings of the survey among Member States............................................................17 7.1. Assessment of the Council Decision ..........................................................................17 7.2. Stages in the functioning of the Council Decision .....................................................18 7.3. National responses to new psychoactive substances ..................................................20 -

A General Approach to the Screening and Confirmation of Tryptamines And
Available online at www.sciencedirect.com Talanta 74 (2008) 512–517 A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation Bo-Hong Chen a, Ju-Tsung Liu b, Wen-Xiong Chen a, Hung-Ming Chen a, Cheng-Huang Lin a,∗ a Department of Chemistry, National Taiwan Normal University, 88 Sec. 4, Tingchow Road, Taipei, Taiwan b Forensic Science Center, Military Police Command, Department of Defense, Taipei, Taiwan Received 6 March 2007; received in revised form 12 June 2007; accepted 13 June 2007 Available online 19 June 2007 Abstract Certain characteristic fragmentations of tryptamines (indoleethylamine) and phenethylamines are described. Based on the GC–EI/MS, LC–ESI/ MS and MALDI/TOFMS, the mass fragmentations of 13 standard compounds, including ␣-methyltryptamine (AMT), N,N-dimethyltryptamine (DMT), 5-methoxy-␣-methyltryptamine (5-MeO-AMT), N,N-diethyltryptamine (DET), N,N-dipropyltryptamine (DPT), N,N-dibutyltryptamine (DBT), N,N-diisopropyltryptamine (DIPT), 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), 5-methoxy-N,N-diisopropyltryptamine (5-MeO- DIPT), methamphetamine (MAMP), 3,4-methylenedioxyamphetamine (3,4-MDA), 3,4-methylenedioxymethamphetamine (3,4-MDMA) and 2- methylamino-1-(3,4-methylenedioxyphenyl)butane (MBDB), were compared. As a result, the parent ions of these analytes were hard to be obtained by GC/MS whereas the protonated molecular ions can be observed clearly by means of ESI/MS and MALDI/TOFMS. Furthermore, two major + + + + characteristic fragmentations, namely and ␣-cleavage ([M +H] → [3-vinylindole] ) and -cleavage ([M +H] → [CH2N RN1RN2]), are produced when the ESI and MALDI modes are used, respectively. In the case of ESI/MS, the fragment obtained from ␣-cleavage is the major process. -

21 CFR Ch. II (4–1–10 Edition) § 1308.12
§ 1308.12 21 CFR Ch. II (4–1–10 Edition) Some trade and other names: N,N- whenever the existence of such salts, Diethyltryptamine; DET isomers, and salts of isomers is possible (18) Dimethyltryptamine ................................................. 7435 Some trade or other names: DMT within the specific chemical designa- (19) 5-methoxy-N,N-diisopropyltryptamine (other tion: name: 5-MeO-DIPT) ................................................... 7439 (1) gamma-hydroxybutyric acid (some other names in- (20) Ibogaine .................................................................. 7260 clude GHB; gamma-hydroxybutyrate; 4- Some trade and other names: 7-Ethyl- hydroxybutyrate; 4-hydroxybutanoic acid; sodium 6,6b,7,8,9,10,12,13-octahydro-2-methoxy-6,9- oxybate; sodium oxybutyrate) .................................... 2010 methano-5H-pyrido [1′, 2′:1,2] azepino [5,4-b] (2) Mecloqualone ........................................................... 2572 indole; Tabernanthe iboga (3) Methaqualone ........................................................... 2565 (21) Lysergic acid diethylamide ..................................... 7315 (22) Marihuana .............................................................. 7360 (f) Stimulants. Unless specifically ex- (23) Mescaline ............................................................... 7381 cepted or unless listed in another (24) Parahexyl—7374; some trade or other names: 3- schedule, any material, compound, Hexyl-1-hydroxy-7,8,9,10-tetrahydro-6,6,9-trimethyl- 6H-dibenzo[b,d]pyran; Synhexyl. mixture, -

LSD), Which Produces Illicit Market in the USA
DEPENDENCE LIABILITY OF "NON-NARCOTIC 9 DRUGS 81 INDOLES The prototype drug in this subgroup (Table XVI) potentials. Ibogaine (S 212) has appeared in the is compound S 219, lysergide (LSD), which produces illicit market in the USA. dependence of the hallucinogen (LSD) type (see above). A tremendous literature on LSD exists which documents fully the dangers of abuse, which REFERENCES is now widespread in the USA, Canada, the United 304. Sandoz Pharmaceuticals Bibliography on Psychoto- Kingdom, Australia and many western European mimetics (1943-1966). Reprinted by the US countries (for references see Table XVI). LSD must Department of Health, Education, & Welfare, be judged as a very dangerous substance which has National Institute of Mental Health, Washington, no established therapeutic use. D.C. 305. Cerletti, A. (1958) In: Heim, R. & Wasson, G. R., Substances S 200-S 203, S 206, S 208, S 213-S 218 ed., Les champignons hallucinogenes du Mexique, and S 220-S 222 are isomers or congeners of LSD. pp. 268-271, Museum national d'Histoire natu- A number of these are much less potent than LSD in relle, Paris (Etude pharmacologique de la hallucinogenic effect or are not hallucinogenic at all psilocybine) (compounds S 203, S 213, S 216, S 217, S 220 and 306. Cohen, S. (1965) The beyond within. The LSD S 222) and accordingly carry a lesser degree of risk story. Atheneum, New York than LSD. None of these weak hallucinogens has 307. Cohen, S. & Ditman, K. S. (1963) Arch. gen. been abused. Other compounds are all sufficiently Psychiat., 8, 475 (Prolonged adverse reactions to potent to make it likely that they would be abused if lysergic acid diethylamide) 308. -

Hallucinogen-Like Actions of 5-Methoxy-N,N-Diisopropyltryptamine in Mice and Rats ⁎ W.E
Pharmacology, Biochemistry and Behavior 83 (2006) 122–129 www.elsevier.com/locate/pharmbiochembeh Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats ⁎ W.E. Fantegrossi a,b, , A.W. Harrington b, C.L. Kiessel b, J.R. Eckler c, R.A. Rabin c, J.C. Winter c, A. Coop d, K.C. Rice e, J.H. Woods b a Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, GA 30322, United States b Department of Pharmacology, University of Michigan Medical School, Ann Arbor, MI, United States c Department of Pharmacology and Toxicology, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY, United States d Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, MD, United States e Laboratory of Medicinal Chemistry, NIDDK, National Institutes of Health, Bethesda, MD Received 2 June 2005; received in revised form 7 December 2005; accepted 29 December 2005 Available online 3 February 2006 Abstract Few studies have examined the effects of 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT) in vivo. In these studies, 5-MeO-DIPT was tested in a drug-elicited head twitch assay in mice where it was compared to the structurally similar hallucinogen N,N-dimethyltryptamine (N,N- DMT) and challenged with the selective serotonin (5-HT)2A antagonist M100907, and in a lysergic acid diethylamide (LSD) discrimination assay in rats where its subjective effects were challenged with M100907 or the 5-HT1A selective antagonist WAY-100635. Finally, the affinity of 5- MeO-DIPT for three distinct 5-HT receptors was determined in rat brain. -
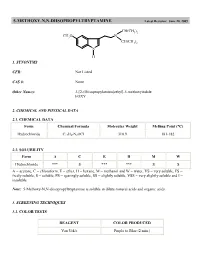
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

Hallucinogens: an Update
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Hallucinogens: An Update 146 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Hallucinogens: An Update Editors: Geraline C. Lin, Ph.D. National Institute on Drug Abuse Richard A. Glennon, Ph.D. Virginia Commonwealth University NIDA Research Monograph 146 1994 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGEMENT This monograph is based on the papers from a technical review on “Hallucinogens: An Update” held on July 13-14, 1992. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company. -

Update of the Generic Definition for Tryptamines
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Zahi Sulaiman 2nd Floor (NW), Seacole Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 1121 [email protected] Norman Baker MP, Minister for Crime Prevention Home Office 2 Marsham Street London SW1P 4DF 10 June 2014 Dear Minister, In December 2013, you commissioned the ACMD to begin a regular review of generic definitions under the Misuse of Drugs Act 1971, with the aim of capturing emerging new psychoactive substances. These drugs are variants of controlled drugs and fall outside the existing scope of the Misuse of Drugs Act 1971. The ACMD has considered evidence available on tryptamines in the context of the Misuse of Drugs Act 1971 and I enclose the Advisory Council’s advice and an expanded definition for tryptamine compounds with this letter. The ACMD’s NPS Committee has firstly reviewed previous research and existing controls to identify those tryptamines now seen to evade the existing controls. The ACMD has also reviewed data provided by the Home Office’s early warning systems and networks, clinical toxicology, prevalence and neuropharmacology in arriving at the expanded generic definition. This expanded generic definition will bring drugs such as alpha-methyltryptamine (AMT) as well as 5-MeO-DALT within the scope of the Misuse of Drugs Act 1971. These are highly potent hallucinogens which act on the 5HT2A receptor, in the same way as LSD. The ACMD therefore recommends that the tryptamines covered by the proposed expanded generic definition in this report, are controlled under the Misuse of Drugs Act (1971) as Class A substances. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo Jose A
Morales-Garcia et al. Translational Psychiatry (2020) 10:331 https://doi.org/10.1038/s41398-020-01011-0 Translational Psychiatry ARTICLE Open Access N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo Jose A. Morales-Garcia 1,2,3,4, Javier Calleja-Conde 5, Jose A. Lopez-Moreno 5, Sandra Alonso-Gil1,2, Marina Sanz-SanCristobal1,2, Jordi Riba6 and Ana Perez-Castillo 1,2,4 Abstract N,N-dimethyltryptamine (DMT) is a component of the ayahuasca brew traditionally used for ritual and therapeutic purposes across several South American countries. Here, we have examined, in vitro and vivo, the potential neurogenic effect of DMT. Our results demonstrate that DMT administration activates the main adult neurogenic niche, the subgranular zone of the dentate gyrus of the hippocampus, promoting newly generated neurons in the granular zone. Moreover, these mice performed better, compared to control non-treated animals, in memory tests, which suggest a functional relevance for the DMT-induced new production of neurons in the hippocampus. Interestingly, the neurogenic effect of DMT appears to involve signaling via sigma-1 receptor (S1R) activation since S1R antagonist blocked the neurogenic effect. Taken together, our results demonstrate that DMT treatment activates the subgranular neurogenic niche regulating the proliferation of neural stem cells, the migration of neuroblasts, and promoting the generation of new neurons in the hippocampus, therefore enhancing adult neurogenesis and -

Test Purchase of New Synthetic Tryptamines Via the Internet: Identity Check by GC-MS and Separation by HPLC
Journal of Applied Pharmaceutical Science Vol. 6 (01), pp. 028-034, January, 2016 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2016.600105 ISSN 2231-3354 Test purchase of new synthetic tryptamines via the Internet: Identity check by GC-MS and separation by HPLC Magdalena Taschwer, Edith Ebner, Martin G Schmid* Department of Pharmaceutical Chemistry, Institute of Pharmaceutical Sciences, University of Graz, Universitätsplatz 1, A-8010 Graz, Austria. ABSTRACT ARTICLE INFO Article history: Over the past few years, a continuous alteration of the recreational drug market took place. Among other novel Received on: 25/09/2015 psychoactive drugs, new synthetic tryptamine derivatives appeared on the market. These compounds are mainly Revised on: 18/10/2015 traded via the Internet, which has become an important marketplace for the sale of recreational drugs. The goal of Accepted on: 08/11/2015 our research was to check, if 13 new synthetic tryptamines obtained by test purchase via different online vendors Available online: 26/01/2016 meet the promised identity. Analysis was performed by GC-MS, using a common 30 m HP-5MS capillary column as stationary phase. Subsequently, a simple HPLC method for the separation of these tryptamines was Key words: developed. Therefore, the aim was to establish a method to separate a broad spectrum of trypamines Tryptamines, Legal highs, simultaneously within short time. Measurements were performed by a LiChrospher® RP-18e column and a Novel psychoactive drugs, mobile phase consisting of 0.1% triethylammonium acetate buffer, methanol and acetonitrile. Both presented HPLC, GC-MS. methods were found to be suitable for the identification as well as separation of tryptamines as the analysis times were short and the selectivity sufficient. -

Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans
molecules Review Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans Paul Cumming 1,2,* , Milan Scheidegger 3 , Dario Dornbierer 3, Mikael Palner 4,5,6 , Boris B. Quednow 3,7 and Chantal Martin-Soelch 8 1 Department of Nuclear Medicine, Bern University Hospital, CH-3010 Bern, Switzerland 2 School of Psychology and Counselling, Queensland University of Technology, Brisbane 4059, Australia 3 Department of Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital of the University of Zurich, CH-8032 Zurich, Switzerland; [email protected] (M.S.); [email protected] (D.D.); [email protected] (B.B.Q.) 4 Odense Department of Clinical Research, University of Southern Denmark, DK-5000 Odense, Denmark; [email protected] 5 Department of Nuclear Medicine, Odense University Hospital, DK-5000 Odense, Denmark 6 Neurobiology Research Unit, Copenhagen University Hospital, DK-2100 Copenhagen, Denmark 7 Neuroscience Center Zurich, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8058 Zurich, Switzerland 8 Department of Psychology, University of Fribourg, CH-1700 Fribourg, Switzerland; [email protected] * Correspondence: [email protected] or [email protected] Abstract: Hallucinogens are a loosely defined group of compounds including LSD, N,N- dimethyltryptamines, mescaline, psilocybin/psilocin, and 2,5-dimethoxy-4-methamphetamine (DOM), Citation: Cumming, P.; Scheidegger, which can evoke intense visual and emotional experiences. We are witnessing a renaissance of re- M.; Dornbierer, D.; Palner, M.; search interest in hallucinogens, driven by increasing awareness of their psychotherapeutic potential. Quednow, B.B.; Martin-Soelch, C. As such, we now present a narrative review of the literature on hallucinogen binding in vitro and Molecular and Functional Imaging ex vivo, and the various molecular imaging studies with positron emission tomography (PET) or Studies of Psychedelic Drug Action in single photon emission computer tomography (SPECT).