Recreational Use, Analysis and Toxicity of Tryptamines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
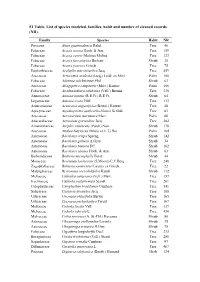
S1 Table. List of Species Modeled, Families, Habit and Number of Cleaned Records (NR)
S1 Table. List of species modeled, families, habit and number of cleaned records (NR). Family Species Habit NR Pinaceae Abies guatemalensis Rehd. Tree 46 Fabaceae Acacia aroma Hook. & Arn. Tree 159 Fabaceae Acacia caven (Molina) Molina Tree 123 Fabaceae Acacia furcatispina Burkart Shrub 35 Fabaceae Acacia praecox Griseb. Tree 75 Euphorbiaceae Acalypha macrostachya Jacq. Tree 459 Arecaceae Acrocomia aculeata (Jacq.) Lodd. ex Mart. Palm 166 Fabaceae Adesmia volckmannii Phil. Shrub 63 Arecaceae Allagoptera campestris (Mart.) Kuntze Palm 106 Fabaceae Anadenanthera colubrina (Vell.) Brenan Tree 330 Annonaceae Annona nutans (R.E.Fr.) R.E.Fr. Shrub 64 Loganiaceae Antonia ovata Pohl Tree 113 Araucariaceae Araucaria angustifolia (Bertol.) Kuntze Tree 48 Apocynaceae Aspidosperma quebracho-blanco Schltdl. Tree 83 Arecaceae Astrocaryum murumuru Mart. Palm 66 Anacardiaceae Astronium graveolens Jacq. Tree 282 Amaranthaceae Atriplex canescens (Pursh) Nutt. Shrub 178 Arecaceae Attalea butyracea (Mutis ex L.f.) We Palm 104 Asteraceae Baccharis crispa Spreng. Shrub 142 Asteraceae Baccharis gilliesii A.Gray Shrub 34 Asteraceae Baccharis trimera DC. Shrub 162 Asteraceae Baccharis ulicina Hook. & Arn. Shrub 63 Berberidaceae Berberis microphylla Forst. Shrub 44 Moraceae Brosimum lactescens (S.Moore) C.C.Berg Tree 248 Zygophyllaceae Bulnesia sarmientoi Lorentz ex Griseb. Tree 22 Malpighiaceae Byrsonima coccolobifolia Kunth Shrub 112 Meliaceae Cabralea canjerana (Vell.) Mart. Tree 157 Icacinaceae Calatola costaricensis Standl. Tree 201 Calophyllaceae Calophyllum brasiliense Cambess. Tree 541 Salicaceae Casearia decandra Jacq. Tree 188 Urticaceae Cecropia obtusifolia Bertol. Tree 165 Urticaceae Cecropia pachystachya Trécul Tree 167 Meliaceae Cedrela fissilis Vell. Tree 137 Meliaceae Cedrela odorata L. Tree 436 Malvaceae Ceiba speciosa (A. St.-Hil.) Ravenna Shrub 80 Asteraceae Chuquiraga avellanedae Lorentz Shrub 35 Asteraceae Chuquiraga erinacea D.Don Shrub 75 Fabaceae Copaifera langsdorffii Desf. -

Anaesthesia and Past Use Of
177 Correspondence were using LSD. It is more popular than other commonly Anaesthesia and past use of used hallucinogens whose quoted incidence of clients are: LSD ketamine 0.1% (super-K/vitamin K.I), psilocybin and psilocin 0.6% (the active alkaloids in the Mexican "magic To the Editor: mushroom"), and 3,4 methylenedioxymethamphetamine We report the case of a 43-yr-old lady who was admitted ~MDMA" 1% (ecstasy). The effects of the concurrent to the Day Surgery Unit for release of her carpal tunnel ingestion of LSD on anaesthesia are well described. 2-4 retinaculum. During the preoperative visit, she reported The long-term effects of the past use of LSD are largely no intercurrent illnesses, drug therapy or allergies. She unknown. We wonder if the hallucinations experienced did say, however, that she was frightened of general anaes- by our patient during anaesthesia were due to her LSD thesia, since she had experienced terrifying dreams during intake many years before. We would be interested to surgery under general anaesthesia on three occasions dur- know if others have had experience anaesthetising patients ing the previous ten years. On further questioning, she who are past users of phencyclidine-derived drugs. admitted that she had used lysergic acid diethylamide (LSD) during the late 1960's, the last occasion being 1968 Geoffrey N. Morris MRCGPFRCA when she had experienced characterstic hallucinations. Patrick T. Magee MSe FRCA She had not experienced hallucinations in the ensuing Anaesthetic Department years, except on the surgical occasions mentioned. Royal United Hospital One of the three previous operations had been per- Combe Park formed at our hospital and the anaesthetic record was Bath BA1 3NG checked. -

Supplementary Materialsupplementary Material
10.1071/BT13149_AC © CSIRO 2013 Australian Journal of Botany 2013, 61(6), 436–445 SUPPLEMENTARY MATERIAL Comparative dating of Acacia: combining fossils and multiple phylogenies to infer ages of clades with poor fossil records Joseph T. MillerA,E, Daniel J. MurphyB, Simon Y. W. HoC, David J. CantrillB and David SeiglerD ACentre for Australian National Biodiversity Research, CSIRO Plant Industry, GPO Box 1600 Canberra, ACT 2601, Australia. BRoyal Botanic Gardens Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia. CSchool of Biological Sciences, Edgeworth David Building, University of Sydney, Sydney, NSW 2006, Australia. DDepartment of Plant Biology, University of Illinois, Urbana, IL 61801, USA. ECorresponding author. Email: [email protected] Table S1 Materials used in the study Taxon Dataset Genbank Acacia abbreviata Maslin 2 3 JF420287 JF420065 JF420395 KC421289 KC796176 JF420499 Acacia adoxa Pedley 2 3 JF420044 AF523076 AF195716 AF195684; AF195703 Acacia ampliceps Maslin 1 KC421930 EU439994 EU811845 Acacia anceps DC. 2 3 JF420244 JF420350 JF419919 JF420130 JF420456 Acacia aneura F.Muell. ex Benth 2 3 JF420259 JF420036 JF420366 JF419935 JF420146 KF048140 Acacia aneura F.Muell. ex Benth. 1 2 3 JF420293 JF420402 KC421323 JQ248740 JF420505 Acacia baeuerlenii Maiden & R.T.Baker 2 3 JF420229 JQ248866 JF420336 JF419909 JF420115 JF420448 Acacia beckleri Tindale 2 3 JF420260 JF420037 JF420367 JF419936 JF420147 JF420473 Acacia cochlearis (Labill.) H.L.Wendl. 2 3 KC283897 KC200719 JQ943314 AF523156 KC284140 KC957934 Acacia cognata Domin 2 3 JF420246 JF420022 JF420352 JF419921 JF420132 JF420458 Acacia cultriformis A.Cunn. ex G.Don 2 3 JF420278 JF420056 JF420387 KC421263 KC796172 JF420494 Acacia cupularis Domin 2 3 JF420247 JF420023 JF420353 JF419922 JF420133 JF420459 Acacia dealbata Link 2 3 JF420269 JF420378 KC421251 KC955787 JF420485 Acacia dealbata Link 2 3 KC283375 KC200761 JQ942686 KC421315 KC284195 Acacia deanei (R.T.Baker) M.B.Welch, Coombs 2 3 JF420294 JF420403 KC421329 KC955795 & McGlynn JF420506 Acacia dempsteri F.Muell. -

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

The Genus Anadenanthera in Amerindian Cultures
THE GENUS ANADENANTHERA IN AMERINDIAN CULTURES BY SIRI VON REIS ALTSCHUL, PH .D. RESEARCH FELLOW BOTANICAL MUSEUM HARVARD UNIVERSITY CAMBRIDGE, MASSACHUSETTS 1972 This monograph is dedicated in affectionate memory to the late DANIEL HERBERT EFRON, PH.D., M.D. 1913-1972 cherished friend of the author and of the Botanical Museum, a true scientist devoted to the interdisciplinary approach in the advancement of knowledge. A/""'f""'<J liz {i>U<// t~ },~u 0<<J4 ~ If;. r:J~ ~ //"'~uI ~ ()< d~ ~ !dtd't;:..1 "./.u.L .A Vdl0 If;;: ~ '" OU'''-k4 :/" tu-d ''''''"-''t2.. ?,,".jd,~ jft I;ft'- ?_rl; A~~ ~r'4tft,t -5 " q,.,<,4 ~~ l' #- /""/) -/~ "1'Ii;. ,1""", "/'/1'1",, I X C"'-r'fttt. #) (../..d ~;, . W,( ~ ~ f;r"'" y it;.,,J 11/" Y 4J.. %~~ l{jr~ t> ~~ ~txh '1'ix r 4 6~" c/<'T'''(''-;{' rn« ?.d ~;;1';;/ a-.d txZ-~ ~ ;o/~ <A.H-iz "" ~".,/( 1-/X< "..< ,:" -.... ~ ~ . JJlr-0? on . .it-(,0.1' r 4 -11<.1.- aw./{') -:JL. P7t;;"j~;1 S .d-At ;0~/lAQ<..t ,ti~?,f,.... vj "7rU<-'- ~I""" =iiR-I1;M~ a....k«<-l, ¢- f!!) d..;.:~ M ~ ~y£/1 ~/.u..-... It'--, "" # :Z:-,k. "i ~ "d/~ efL<.<~/ ,w 1'#,') /';~~;d-t a;.. tlArl-<7'" I .Ii;'~.1 (1(-;.,} >Lc -(l"7C),.,..,;.. :.... ,,:/ ~ /-V,~ , ,1" # (i F'"' l' fJ~~A- (.tG- ~/~ Z:--7Co- ,,:. ,L7r= f,-, , ~t) ahd-p;: fJ~ / tr>d .4 ~f- $. b".,,1 ~/. ~ pd. 1'7'-· X ~-t;;;;.,~;z jM ~0Y:tJ;; ~ """.,4? br;K,' ./.n.u" ~ 7r .".,.~,j~ ;;f;tT ~ ..4'./ ;pf,., tJd~ M_~ (./I<'/~.'. IU. et. c./,. ~L.y !f-t.<H>:t;.tu ~ ~,:,-,p., .....:. -

5-Meo-DMT) in a Journal of Psychedelic Studies Naturalistic Setting
A comparison of reactivation experiences following vaporization and intramuscular injection (IM) of synthetic 5-methoxy-N,N- dimethyltryptamine (5-MeO-DMT) in a Journal of Psychedelic Studies naturalistic setting 4 (2020) 2, 104–113 1p 2 DOI: MALIN V. UTHAUG , R. LANCELOTTA , 3 4,5 10.1556/2054.2020.00123 A. M. ORTIZ BERNAL , A. K. DAVIS and p © 2020 The Author(s) JOHANNES G. RAMAEKERS1 1 Department of Neuropsychology and Psychopharmacology, Faculty of Psychology and Neuroscience, Maastricht University, The Netherlands 2 ORIGINAL ARTICLE Innate Path, Lakewood, CO, USA 3 School of Human Ecology, Human Development and Family Studies, University of Wisconsin, Madison, WI, USA 4 College of Social Work, The Ohio State University, Columbus, 43210, OH, USA 5 Center for Psychedelic and Consciousness Research, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University, Baltimore, 21224, MD, USA Received: November 18, 2019 • Accepted: December 22, 2019 Published online: March 26, 2020 ABSTRACT Background: Previous research suggests a therapeutic potential of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT). However, online anecdotal reports have described a phenomenon following cessation of the acute effects of 5-MeO-DMT use which has been termed reactivation (i.e., re-experiencing [“flashback”]). To date, no research has investigated whether different routes of administration may confer different reactivation rates, effects and experiences. Aims: We aimed to assess whether intramuscular injection (IM) and vaporization of 5-MeO-DMT conferred different reactivation rates, changes in satisfaction with life as well as ratings of the experience with ego dissolution and the mystical. Methods: Using internet-based advertisements, 27 respondents (Mage = 32. -

Long-Lasting Analgesic Effect of the Psychedelic Drug Changa: a Case Report
CASE REPORT Journal of Psychedelic Studies 3(1), pp. 7–13 (2019) DOI: 10.1556/2054.2019.001 First published online February 12, 2019 Long-lasting analgesic effect of the psychedelic drug changa: A case report GENÍS ONA1* and SEBASTIÁN TRONCOSO2 1Department of Anthropology, Philosophy and Social Work, Universitat Rovira i Virgili, Tarragona, Spain 2Independent Researcher (Received: August 23, 2018; accepted: January 8, 2019) Background and aims: Pain is the most prevalent symptom of a health condition, and it is inappropriately treated in many cases. Here, we present a case report in which we observe a long-lasting analgesic effect produced by changa,a psychedelic drug that contains the psychoactive N,N-dimethyltryptamine and ground seeds of Peganum harmala, which are rich in β-carbolines. Methods: We describe the case and offer a brief review of supportive findings. Results: A long-lasting analgesic effect after the use of changa was reported. Possible analgesic mechanisms are discussed. We suggest that both pharmacological and non-pharmacological factors could be involved. Conclusion: These findings offer preliminary evidence of the analgesic effect of changa, but due to its complex pharmacological actions, involving many neurotransmitter systems, further research is needed in order to establish the specific mechanisms at work. Keywords: analgesic, pain, psychedelic, psychoactive, DMT, β-carboline alkaloids INTRODUCTION effects of ayahuasca usually last between 3 and 5 hr (McKenna & Riba, 2015), but the effects of smoked changa – The treatment of pain is one of the most significant chal- last about 15 30 min (Ott, 1994). lenges in the history of medicine. At present, there are still many challenges that hamper pain’s appropriate treatment, as recently stated by American Pain Society (Gereau et al., CASE DESCRIPTION 2014). -

EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011)
EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011) 912 final COMMISSION STAFF WORKING PAPER on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances Accompanying the document REPORT FROM THE COMMISSION on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances {COM(2011) 430 final} EN EN TABLE OF CONTENTS 1. Introduction...................................................................................................................3 2. Methodology.................................................................................................................4 3. Key findings from the 2002 evaluation of the Joint Action on synthetic drugs ...........5 4. Overview of notifications, types of substances and trends at EU level 2005-2010......7 5. Other EU legislation relevant for the regulation of new psychoactive substances.....12 6. Functioning of the Council Decision on new psychoactive substances .....................16 7. Findings of the survey among Member States............................................................17 7.1. Assessment of the Council Decision ..........................................................................17 7.2. Stages in the functioning of the Council Decision .....................................................18 7.3. National responses to new psychoactive substances ..................................................20 -

Soma and Haoma: Ayahuasca Analogues from the Late Bronze Age
ORIGINAL ARTICLE Journal of Psychedelic Studies 3(2), pp. 104–116 (2019) DOI: 10.1556/2054.2019.013 First published online July 25, 2019 Soma and Haoma: Ayahuasca analogues from the Late Bronze Age MATTHEW CLARK* School of Oriental and African Studies (SOAS), Department of Languages, Cultures and Linguistics, University of London, London, UK (Received: October 19, 2018; accepted: March 14, 2019) In this article, the origins of the cult of the ritual drink known as soma/haoma are explored. Various shortcomings of the main botanical candidates that have so far been proposed for this so-called “nectar of immortality” are assessed. Attention is brought to a variety of plants identified as soma/haoma in ancient Asian literature. Some of these plants are included in complex formulas and are sources of dimethyl tryptamine, monoamine oxidase inhibitors, and other psychedelic substances. It is suggested that through trial and error the same kinds of formulas that are used to make ayahuasca in South America were developed in antiquity in Central Asia and that the knowledge of the psychoactive properties of certain plants spreads through migrants from Central Asia to Persia and India. This article summarizes the main arguments for the botanical identity of soma/haoma, which is presented in my book, The Tawny One: Soma, Haoma and Ayahuasca (Muswell Hill Press, London/New York). However, in this article, all the topics dealt with in that publication, such as the possible ingredients of the potion used in Greek mystery rites, an extensive discussion of cannabis, or criteria that we might use to demarcate non-ordinary states of consciousness, have not been elaborated. -

Review of Psilocybin/Psilocin Pharmacology Isaac Dehart
Review of Psilocybin/Psilocin Pharmacology Isaac DeHart Introduction Psilocybin (PY, 4-phosphoryloxy-N,N-dimethyltryptamine or O-phosphoryl-4-hydroxy- N,N-dimethyltryptamine) (Figure 1, 1) is an indolamine (Figure 1, 2), and the primary ingredient in magic mushrooms. It has been implicated as a treatment for a number of psychological ailments including depression, general anxiety disorder, various addictions, obsessive- compulsive disorder, and post-traumatic stress disorder.1–4 Because of the drug’s re-emerging relevance in clinical applications, and because of its distinctive and informative effects on brain function, it is important to understand the pharmacology and general chemistry underlying its metabolism. Psilocybin is a prodrug of psilocin Magic mushrooms, the natural source of psilocybin may refer to several different hallucination-causing fungi, but the most potent of these are found in the genus Psilocybe. When ingested, magic mushrooms cause “trips,” wherein a user experiences euphoria, colorful hallucinations, and changes in perception, cognition and mood. In the case of “bad trips,” a user may experience states of intense panic or paranoia.5,6 More generally, these trips are sometimes described in terms of psychotic states, and behavioral studies involving psilocybin have demonstrated similarities between the effects of psilocybin and schizophrenia both in subjective experience and in physiological response.7 While psilocybin is more widely known as the cause behind these effects, it is referred to by researchers as a prodrug, -

Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics
TESI DOCTORAL HUMAN PHARMACOLOGY OF AYAHUASCA JORDI RIBA Barcelona, 2003 Director de la Tesi: DR. MANEL JOSEP BARBANOJ RODRÍGUEZ A la Núria, el Marc i l’Emma. No pasaremos en silencio una de las cosas que á nuestro modo de ver llamará la atención... toman un bejuco llamado Ayahuasca (bejuco de muerto ó almas) del cual hacen un lijero cocimiento...esta bebida es narcótica, como debe suponerse, i á pocos momentos empieza a producir los mas raros fenómenos...Yo, por mí, sé decir que cuando he tomado el Ayahuasca he sentido rodeos de cabeza, luego un viaje aéreo en el que recuerdo percibia las prespectivas mas deliciosas, grandes ciudades, elevadas torres, hermosos parques i otros objetos bellísimos; luego me figuraba abandonado en un bosque i acometido de algunas fieras, de las que me defendia; en seguida tenia sensación fuerte de sueño del cual recordaba con dolor i pesadez de cabeza, i algunas veces mal estar general. Manuel Villavicencio Geografía de la República del Ecuador (1858) Das, was den Indianer den “Aya-huasca-Trank” lieben macht, sind, abgesehen von den Traumgesichten, die auf sein persönliches Glück Bezug habenden Bilder, die sein inneres Auge während des narkotischen Zustandes schaut. Louis Lewin Phantastica (1927) Agraïments La present tesi doctoral constitueix la fase final d’una idea nascuda ara fa gairebé nou anys. El fet que aquest treball sobre la farmacologia humana de l’ayahuasca hagi estat una realitat es deu fonamentalment al suport constant del seu director, el Manel Barbanoj. Voldria expressar-li la meva gratitud pel seu recolzament entusiàstic d’aquest projecte, molt allunyat, per la natura del fàrmac objecte d’estudi, dels que fins al moment s’havien dut a terme a l’Àrea d’Investigació Farmacològica de l’Hospital de Sant Pau. -

Psilocybin Mushrooms Fact Sheet
Psilocybin Mushrooms Fact Sheet January 2017 What are psilocybin, or “magic,” mushrooms? For the next two decades thousands of doses of psilocybin were administered in clinical experiments. Psilocybin is the main ingredient found in several types Psychiatrists, scientists and mental health of psychoactive mushrooms, making it perhaps the professionals considered psychedelics like psilocybin i best-known naturally-occurring psychedelic drug. to be promising treatments as an aid to therapy for a Although psilocybin is considered active at doses broad range of psychiatric diagnoses, including around 3-4 mg, a common dose used in clinical alcoholism, schizophrenia, autism spectrum disorders, ii,iii,iv research settings ranges from 14-30 mg. Its obsessive-compulsive disorder, and depression.xiii effects on the brain are attributed to its active Many more people were also introduced to psilocybin metabolite, psilocin. Psilocybin is most commonly mushrooms and other psychedelics as part of various found in wild or homegrown mushrooms and sold religious or spiritual practices, for mental and either fresh or dried. The most popular species of emotional exploration, or to enhance wellness and psilocybin mushrooms is Psilocybe cubensis, which is creativity.xiv usually taken orally either by eating dried caps and stems or steeped in hot water and drunk as a tea, with Despite this long history and ongoing research into its v a common dose around 1-2.5 grams. therapeutic and medical benefits,xv since 1970 psilocybin and psilocin have been listed in Schedule I of the Controlled Substances Act, the most heavily Scientists and mental health professionals criminalized category for drugs considered to have a consider psychedelics like psilocybin to be “high potential for abuse” and no currently accepted promising treatments as an aid to therapy for a medical use – though when it comes to psilocybin broad range of psychiatric diagnoses.